2 - School of Physics
... An example from hep An example from hep • SM without higgs predicts that after a set of cuts 64 events will remain, but in reality 80 events are found, is it the higgs signal? • No, because 80‐64 = 16 = 2 * sqrt(64) • Remember that RMS for a poisson distribution is sq ( ea ) sqrt(mean) ...
... An example from hep An example from hep • SM without higgs predicts that after a set of cuts 64 events will remain, but in reality 80 events are found, is it the higgs signal? • No, because 80‐64 = 16 = 2 * sqrt(64) • Remember that RMS for a poisson distribution is sq ( ea ) sqrt(mean) ...
Lecture 7
... Problem 2 : Given x0,...,xN-1 with a guarantee that there is exactly one xi=1, find i Problem 3: Given x0,...,xN-1, compute OR(x0,...,xN-1) Problem 4: Given x0,...,xN-1 with guarantee that either exactly one xi=1 or no xi=1, decide which 2),3) are easier than 1), and 4) is easier than 2),3) ...
... Problem 2 : Given x0,...,xN-1 with a guarantee that there is exactly one xi=1, find i Problem 3: Given x0,...,xN-1, compute OR(x0,...,xN-1) Problem 4: Given x0,...,xN-1 with guarantee that either exactly one xi=1 or no xi=1, decide which 2),3) are easier than 1), and 4) is easier than 2),3) ...
Name
... Example 4.12: The hockey puck in the diagram struck by a hockey stick, is given an initial speed of 20.0 m/s on a frozen pone. The puck remains on the ice and slides 1.20 x 102 m, slowing down steadily until it comes to rest. Determine the coefficient of kinetic friction between the puck and the ice ...
... Example 4.12: The hockey puck in the diagram struck by a hockey stick, is given an initial speed of 20.0 m/s on a frozen pone. The puck remains on the ice and slides 1.20 x 102 m, slowing down steadily until it comes to rest. Determine the coefficient of kinetic friction between the puck and the ice ...
Quantum effects in energy and charge transfer in an
... defined in Eq. (7). Here, we use the fact that the Hamiltonian H in Eq. (6) is also expressed in terms of the operators ρμν taken at the same moment of time t. For two of these operators, ρμν (t) and ραβ (t), we have simple multiplication rules: ρμν ραβ = δνα ρμβ . These rules allow to calculate com ...
... defined in Eq. (7). Here, we use the fact that the Hamiltonian H in Eq. (6) is also expressed in terms of the operators ρμν taken at the same moment of time t. For two of these operators, ρμν (t) and ραβ (t), we have simple multiplication rules: ρμν ραβ = δνα ρμβ . These rules allow to calculate com ...
Comment on “Quantum Monte Carlo Approach to Elementary
... by Haldane [2]. Using chain lengths up to L 128, he estimates the gap to be 0.049(18). This result is compared to the findings of other authors [3] and disagrees substantially from our result [4] 0.085(5). In this Comment, we would like to point out why we feel this result to be inconsistent with ...
... by Haldane [2]. Using chain lengths up to L 128, he estimates the gap to be 0.049(18). This result is compared to the findings of other authors [3] and disagrees substantially from our result [4] 0.085(5). In this Comment, we would like to point out why we feel this result to be inconsistent with ...
論文の構成 - 秋山研究室
... At intermidiate ne, the peak of trion stays at the same energy with ne while the absorption peak at high ne blue-shifts rapidly from the higher energy side of the trion peak. In other words, the absorption peak at high ne does not originate from the trion peak. This is interesting because, in 2D ele ...
... At intermidiate ne, the peak of trion stays at the same energy with ne while the absorption peak at high ne blue-shifts rapidly from the higher energy side of the trion peak. In other words, the absorption peak at high ne does not originate from the trion peak. This is interesting because, in 2D ele ...
Chapter 6 Electronic Structure of Atoms
... Energies of Orbitals • As the number of electrons increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. Electronic Structure of Atoms ...
... Energies of Orbitals • As the number of electrons increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. Electronic Structure of Atoms ...
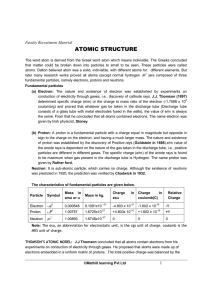
atomic structure
... Isotopes: Atoms of same element which have same atomic number but different mass numbers are called isotopes Example: Cl atomic weight is 35.5 due to existence of Cl 35 and Cl 37 , which are present in the ratio of 3:1 in nature Most of our information about the arrangement of electrons in atoms has ...
... Isotopes: Atoms of same element which have same atomic number but different mass numbers are called isotopes Example: Cl atomic weight is 35.5 due to existence of Cl 35 and Cl 37 , which are present in the ratio of 3:1 in nature Most of our information about the arrangement of electrons in atoms has ...