Read "FDA Violation of the Rule of Law"
... solvent. That is anti-freeze. Although Massengill was convicted of gross negligence, the Roosevelt administration, feeling its oats after the "switch in time that saved nine," called for adoption of the Food, Drug and C osmetic Act of 1938. That law required companies to submit New Drug Applications ...
... solvent. That is anti-freeze. Although Massengill was convicted of gross negligence, the Roosevelt administration, feeling its oats after the "switch in time that saved nine," called for adoption of the Food, Drug and C osmetic Act of 1938. That law required companies to submit New Drug Applications ...
DESIGNER DRUG AWARENESS PROJECT
... supplements for weight loss have not been studied in randomized controlled trials in humans. ...
... supplements for weight loss have not been studied in randomized controlled trials in humans. ...
FDA Supplement - Oregon State University Research Office
... considered to be drugs when they are used to diagnose, cure, mitigate, treat, or prevent disease. Under FDA regulations, research that involves use of a drug other than the use of a marketed drug in the course of medical practice must have an Investigational New Drug (IND) Application, unless the st ...
... considered to be drugs when they are used to diagnose, cure, mitigate, treat, or prevent disease. Under FDA regulations, research that involves use of a drug other than the use of a marketed drug in the course of medical practice must have an Investigational New Drug (IND) Application, unless the st ...
Moore - AL.com
... patients with atrial fibrillation such as the Moore. There are, however, strict FDA regulations about the form and content of such promotion. In fact, it is unlawful for a manufacturer to promote any drug for a use not described in the approved labeling of the drug. Both Defendants by their various ...
... patients with atrial fibrillation such as the Moore. There are, however, strict FDA regulations about the form and content of such promotion. In fact, it is unlawful for a manufacturer to promote any drug for a use not described in the approved labeling of the drug. Both Defendants by their various ...
Complaint 1
... specifically instructed that promotional materials for Actiq that had not been approved by FDA for use in marketing, but which were nevertheless used in making "details" to physicians, should not be left with physicians. Defendant's Actiq sales representatives were further reminded to be cautious ab ...
... specifically instructed that promotional materials for Actiq that had not been approved by FDA for use in marketing, but which were nevertheless used in making "details" to physicians, should not be left with physicians. Defendant's Actiq sales representatives were further reminded to be cautious ab ...
July 2012 - Kaiser Permanente
... plan renews o For example, if a plan renews on November 2, the member would pay their current copays for contraceptives between August 1 and November 1 and would pay $0 copay after November 1 • If HMO member receives a formulary exception for a nonformulary contraceptive, it will be covered at $0 co ...
... plan renews o For example, if a plan renews on November 2, the member would pay their current copays for contraceptives between August 1 and November 1 and would pay $0 copay after November 1 • If HMO member receives a formulary exception for a nonformulary contraceptive, it will be covered at $0 co ...
Novartis Pharmaceuticals to Pay $390 Million
... Inc., have already agreed to pay $15 million and $60 million, respectively, to resolve claims that they accepted kickbacks from Novartis to promote Exjade. Novartis, which is headquartered in East Hanover, New Jersey, is a subsidiary of the Swiss pharmaceutical company Novartis AG. In late 2005, Exj ...
... Inc., have already agreed to pay $15 million and $60 million, respectively, to resolve claims that they accepted kickbacks from Novartis to promote Exjade. Novartis, which is headquartered in East Hanover, New Jersey, is a subsidiary of the Swiss pharmaceutical company Novartis AG. In late 2005, Exj ...
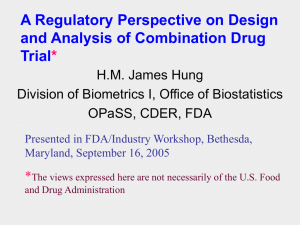
P13_JHung_CombProducts
... 1) Assert that the combination drug is more effective than each component drug alone 2) Obtain useful and reliable DR information - identify a dose range where effect increases as a function of dose - identify a dose beyond which there is no appreciable increase of the effect or undesirable ...
... 1) Assert that the combination drug is more effective than each component drug alone 2) Obtain useful and reliable DR information - identify a dose range where effect increases as a function of dose - identify a dose beyond which there is no appreciable increase of the effect or undesirable ...
DEC 22 1994 John Spector Caprice-Greystoke, Ltd.
... The agency's review of all of these data was completed within the last year. This review has also led to a determination that the existing data are inadequate to.support immediate-release doses of 25 to 37.5 mg PPA because adequate clinical studies were not submitted to support the effectiveness of ...
... The agency's review of all of these data was completed within the last year. This review has also led to a determination that the existing data are inadequate to.support immediate-release doses of 25 to 37.5 mg PPA because adequate clinical studies were not submitted to support the effectiveness of ...
FDA accepts for filing a New Drug Application (NDA) for
... “prescription drug user fee act” goal date set for 30 August 2007. Subject to the approval of the drug by the FDA, Ipsen’s partner Tercica will market Somatuline® Autogel® in the United States. Somatuline® Autogel® has already received a marketing approval in Canada on 17 July 2006, and is currently ...
... “prescription drug user fee act” goal date set for 30 August 2007. Subject to the approval of the drug by the FDA, Ipsen’s partner Tercica will market Somatuline® Autogel® in the United States. Somatuline® Autogel® has already received a marketing approval in Canada on 17 July 2006, and is currently ...
J=” m -1- w
... experiences with IGF-I. IGF-I is a recombinant biological product; however, this approval process was taken by CDER that requires two independent clinical trials. All other neurotrophic factors, such as CNTF, BDNF, or GDNF, were to be evaluated by CBER that requires only one clinical trial. I do not ...
... experiences with IGF-I. IGF-I is a recombinant biological product; however, this approval process was taken by CDER that requires two independent clinical trials. All other neurotrophic factors, such as CNTF, BDNF, or GDNF, were to be evaluated by CBER that requires only one clinical trial. I do not ...
navigating the fda
... comparison if applicable, suggestion on how to be classified, indications for use, protocol outline with study endpoints, suggest N size. If drug results of animal study safety, pilot/lab, feasibility results, statistical methods to be used • List of questions for FDA ...
... comparison if applicable, suggestion on how to be classified, indications for use, protocol outline with study endpoints, suggest N size. If drug results of animal study safety, pilot/lab, feasibility results, statistical methods to be used • List of questions for FDA ...
News Virginia Board of Pharmacy
... Current pharmacist licenses and pharmacy technician registrations expire at midnight on December 31, 2014. Please note that practicing on a lapsed license or registration is unlawful and constitutes grounds for disciplinary action by the Virginia Board of Pharmacy. Renewal notification letters will ...
... Current pharmacist licenses and pharmacy technician registrations expire at midnight on December 31, 2014. Please note that practicing on a lapsed license or registration is unlawful and constitutes grounds for disciplinary action by the Virginia Board of Pharmacy. Renewal notification letters will ...
AAPM Report No 121
... Medical products, devices, drugs or biologics must be cleared, approved, or licensed by the U.S. Food and Drug Administration (FDA) prior to being legally marketed in the United States. The terms clearance, approval, and licensing depend on the type of medical product, and which of the FDA Centers c ...
... Medical products, devices, drugs or biologics must be cleared, approved, or licensed by the U.S. Food and Drug Administration (FDA) prior to being legally marketed in the United States. The terms clearance, approval, and licensing depend on the type of medical product, and which of the FDA Centers c ...
AAJ Unequal Harm - The American Association For Justice
... n 1978, 34-year-old nurse Margaret Worsham was hospitalized for a pelvic infection. Over the next several days her condition worsened until she was forced to undergo a complete hysterectomy. Worsham had suffered a tubo-ovarian abcess, caused by a contraceptive intrauterine device (IUD) – the Dalkon ...
... n 1978, 34-year-old nurse Margaret Worsham was hospitalized for a pelvic infection. Over the next several days her condition worsened until she was forced to undergo a complete hysterectomy. Worsham had suffered a tubo-ovarian abcess, caused by a contraceptive intrauterine device (IUD) – the Dalkon ...
Draft Guidance Product Liability Implications
... Although the Draft Guidance contains only non-binding recommendations, it provides some indication on the FDA’s current views relating to biosimilar labeling. The highlights of the Draft Guidance recommendations are as follows: Overall Approach. The FDA anticipates the text in a biosimilar label b ...
... Although the Draft Guidance contains only non-binding recommendations, it provides some indication on the FDA’s current views relating to biosimilar labeling. The highlights of the Draft Guidance recommendations are as follows: Overall Approach. The FDA anticipates the text in a biosimilar label b ...
AMPHETAMINES RATIONALE FOR INCLUSION IN PA PROGRAM
... Amphetamine is a CNS stimulant and DEA schedule II drug, which is FDA approved for attention deficit hyperactivity disorder (ADHD) and narcolepsy. The exact mechanism by which amphetamines exert their action is unknown; however amphetamines are thought to block the reuptake of norepinephrine and dop ...
... Amphetamine is a CNS stimulant and DEA schedule II drug, which is FDA approved for attention deficit hyperactivity disorder (ADHD) and narcolepsy. The exact mechanism by which amphetamines exert their action is unknown; however amphetamines are thought to block the reuptake of norepinephrine and dop ...
Analytical Method Validation in Early Drug
... • The API manufacturing process and the dosage form may be evolving/improving • The validation parameters may be reduced during early development with gradual addition as the IND proceeds to an ANDA/BLA/NDA • Validation data should be retained to link analytical procedures used in early phase/pivota ...
... • The API manufacturing process and the dosage form may be evolving/improving • The validation parameters may be reduced during early development with gradual addition as the IND proceeds to an ANDA/BLA/NDA • Validation data should be retained to link analytical procedures used in early phase/pivota ...
Antibiotic Use in Agriculture: Background and Legislation CRS Report for Congress
... Use of Antibiotics in Agriculture8 Types of Use Antibiotics are used in food-producing animals for three major reasons, according to HHS’s Centers for Disease Control and Prevention (CDC).9 First, they are used in high doses for short periods of time to treat sick animals. Second, they are used—also ...
... Use of Antibiotics in Agriculture8 Types of Use Antibiotics are used in food-producing animals for three major reasons, according to HHS’s Centers for Disease Control and Prevention (CDC).9 First, they are used in high doses for short periods of time to treat sick animals. Second, they are used—also ...
Provision or distribution of growth hormone for
... esis. Transgenic mice that produce supraphysiological levels of GH for their age have markedly reduced life spans and experience premature onset of age-related cognitive changes.31 Rats with adult-onset GHD and decreased IGF-1 levels have a 30% decrease in tumor incidence and a 16% decrease in disea ...
... esis. Transgenic mice that produce supraphysiological levels of GH for their age have markedly reduced life spans and experience premature onset of age-related cognitive changes.31 Rats with adult-onset GHD and decreased IGF-1 levels have a 30% decrease in tumor incidence and a 16% decrease in disea ...
MedDRA Use at FDA
... Calculation of reporting rate in the exposed population – using denominator (drug use data) Compare to estimates of background occurrence rate in the general population Comparison of a similar period for similar or the same class of products Analyses of cases to identify population at risk Studying ...
... Calculation of reporting rate in the exposed population – using denominator (drug use data) Compare to estimates of background occurrence rate in the general population Comparison of a similar period for similar or the same class of products Analyses of cases to identify population at risk Studying ...
September 2015 - Institute For Safe Medication Practices
... process that may take years to complete. When legal claims reach a drug manufacturer they are also reported to the FDA as adverse events. In 2014, the biggest reported litigation target (n = 4,727) was the cholesterol-lowering drug atorvastatin (LIPITOR), and the issue was whether it causes diabetes ...
... process that may take years to complete. When legal claims reach a drug manufacturer they are also reported to the FDA as adverse events. In 2014, the biggest reported litigation target (n = 4,727) was the cholesterol-lowering drug atorvastatin (LIPITOR), and the issue was whether it causes diabetes ...
Gabapentin Fact Sheet - The Main Line Center for the Family
... Neurontin (gabapentin) is better known for its use as an anticonvulsant—a medication for treating epilepsy. This may present some confusion for patients, as well as their families, when they are prescribed Neurontin without a history of seizures. Although Neurontin is approved by the U.S. Food and D ...
... Neurontin (gabapentin) is better known for its use as an anticonvulsant—a medication for treating epilepsy. This may present some confusion for patients, as well as their families, when they are prescribed Neurontin without a history of seizures. Although Neurontin is approved by the U.S. Food and D ...
\
... petition submitted by you on behalf of Caprice-Greystoke,Ltd., requesting that the Food and Drug Administration (FDA) (1) open the administrative record to include certain studies on “Spray-U-Thin, ” an over-the-counter (OTC) oral liquid immediate-releaseappetite suppressant containingphenylpropanol ...
... petition submitted by you on behalf of Caprice-Greystoke,Ltd., requesting that the Food and Drug Administration (FDA) (1) open the administrative record to include certain studies on “Spray-U-Thin, ” an over-the-counter (OTC) oral liquid immediate-releaseappetite suppressant containingphenylpropanol ...