
Thermochemical Investigations of Nearly Ideal
... Iodine was Matheson Coleman and Bell Certified ACS Reagent Grade. Stannic iodide was prepared by refluxing metallic tin powder with iodine in chloroform as suggested by Wheatland." The product was recrystallized three times from hot chloroform, giving a melting point of 144 f 1 "C (lit. mp 144.5 "C) ...
... Iodine was Matheson Coleman and Bell Certified ACS Reagent Grade. Stannic iodide was prepared by refluxing metallic tin powder with iodine in chloroform as suggested by Wheatland." The product was recrystallized three times from hot chloroform, giving a melting point of 144 f 1 "C (lit. mp 144.5 "C) ...
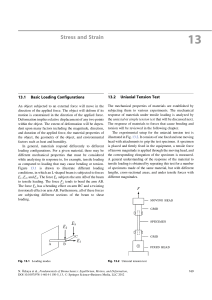
Stress and Strain
... subjected to tensile loading. Let Dl1 be the amount of elongation measured between A and B, and Dl2 be the increase in length between C and D. Dl1 and Dl2 are certainly some measures of deformation. However, they depend on the respective gage lengths, such that Dl1 >Dl2 . On the other hand, if the r ...
... subjected to tensile loading. Let Dl1 be the amount of elongation measured between A and B, and Dl2 be the increase in length between C and D. Dl1 and Dl2 are certainly some measures of deformation. However, they depend on the respective gage lengths, such that Dl1 >Dl2 . On the other hand, if the r ...
The decomposition of hydrogen peroxide to form water and oxygen
... clearly show the method used and the steps involved in arriving at your answers. It is to your advantage to do this, since you may obtain partial credit if you do and you will receive little or no credit if you do not. ...
... clearly show the method used and the steps involved in arriving at your answers. It is to your advantage to do this, since you may obtain partial credit if you do and you will receive little or no credit if you do not. ...
SOLUBILITY RULES FOR IONIC COMPOUNDS IN WATER
... (a) Calculate the moles of each of the ions present in the solution before any reaction has occurred. (b) Calculate the moles of each of the ions present in the solution after any reaction has occurred. (c) Calculate the molarities of each of the ions present in the solution after any reaction has o ...
... (a) Calculate the moles of each of the ions present in the solution before any reaction has occurred. (b) Calculate the moles of each of the ions present in the solution after any reaction has occurred. (c) Calculate the molarities of each of the ions present in the solution after any reaction has o ...
Preparation and characterization of phosphate and arsenate
... bubbled through the precipitation medium to prevent the formation of carbonate apatite and also to keep the medium well stirred. The produce was refluxed for about 2 h in contact with the mother-liquor, left overnight, filtered through a G4 sintered glass crucible and washed with water until the was ...
... bubbled through the precipitation medium to prevent the formation of carbonate apatite and also to keep the medium well stirred. The produce was refluxed for about 2 h in contact with the mother-liquor, left overnight, filtered through a G4 sintered glass crucible and washed with water until the was ...
Keq = [A] [B] [C] [D]
... Only one of these values will be possible. Looking back at our data, we started with only 4.0 mol/L of H2 . If we subtract 4.7 mol/L, we will get a negative value for concentration, which is impossible. Therefore, x=4.7 is not a realistic answer, so x=1.9 mol/L. Substitute 'x =1.9 mol/L' back up int ...
... Only one of these values will be possible. Looking back at our data, we started with only 4.0 mol/L of H2 . If we subtract 4.7 mol/L, we will get a negative value for concentration, which is impossible. Therefore, x=4.7 is not a realistic answer, so x=1.9 mol/L. Substitute 'x =1.9 mol/L' back up int ...
AP Chemistry Review Preparing for the AP
... Give examples and solve calculation problems related to each of the three theories. Sketch a cathode ray tube as demonstrated in class and state how J.J. Thomson’s experiments led to the idea that atoms have positive and negative parts, the negative parts are all the same, and the negative parts ...
... Give examples and solve calculation problems related to each of the three theories. Sketch a cathode ray tube as demonstrated in class and state how J.J. Thomson’s experiments led to the idea that atoms have positive and negative parts, the negative parts are all the same, and the negative parts ...
Genericity of confined chemical garden patterns with regard to
... between these two patterns probably being continuous. Additional experiments will be needed to define more accurately the borders of each region of this diagram. Nevertheless, it is already useful to guide future experiments with which this map may be refined. The formation of chemical gardens takes ...
... between these two patterns probably being continuous. Additional experiments will be needed to define more accurately the borders of each region of this diagram. Nevertheless, it is already useful to guide future experiments with which this map may be refined. The formation of chemical gardens takes ...
Phase Polymorphism of [Mn(DMSO) ](ClO ) Studied by
... phase K0. On the DSC curve No 8 we can see one slight anomaly at TC4 , connected with the phase transition metastable phase KIII ↔ metastable phase KII, one major anomaly at TC3 , connected with the phase transition stable phase KIb → stable phase KIa and a slight one at TC1 , connected with the pha ...
... phase K0. On the DSC curve No 8 we can see one slight anomaly at TC4 , connected with the phase transition metastable phase KIII ↔ metastable phase KII, one major anomaly at TC3 , connected with the phase transition stable phase KIb → stable phase KIa and a slight one at TC1 , connected with the pha ...
Ch. 14 Study Guide
... 19. When excess solid is added to a solution, an equilibrium is set up between the dissolution and deposition processes. If an equilibrium is established, the solution is said to be saturated. 20. If an equilibrium cannot be established, then the solution is unsaturated. 21. Supersaturated solutions ...
... 19. When excess solid is added to a solution, an equilibrium is set up between the dissolution and deposition processes. If an equilibrium is established, the solution is said to be saturated. 20. If an equilibrium cannot be established, then the solution is unsaturated. 21. Supersaturated solutions ...
Spinodal decomposition

Spinodal decomposition is a mechanism for the rapid unmixing of a mixture of liquids or solids from one thermodynamic phase, to form two coexisting phases. As an example, consider a hot mixture of water and an oil. At high temperatures the oil and the water may mix to form a single thermodynamic phase in which water molecules are surrounded by oil molecules and vice versa. The mixture is then suddenly cooled to a temperature at which thermodynamic equilibrium favours an oil-rich phase coexisting with a water-rich phase. Spinodal decomposition then occurs when the mixture is such that there is essentially no barrier to nucleation of the new oil-rich and water-rich phases. In other words, the oil and water molecules immediately start to cluster together into microscopic water-rich and oil-rich clusters throughout the liquid. These clusters then rapidly grow and coalesce until there is a single macroscopic oil-rich cluster, the oil-rich phase, and a single water-rich cluster, the water-rich phase.Spinodal decomposition can be contrasted with nucleation and growth. There the initial formation of the microscopic clusters involves a large free energy barrier, and so can be very slow, and may occur as little as once in the initial phase, not throughout the phase, as happens in spinodal decomposition.Spinodal decomposition is of interest for two primary reasons. In the first place, it is one of the few phase transformations in solids for which there is any plausible quantitative theory. The reason for this is the inherent simplicity of the reaction. Since there is no thermodynamic barrier to the reaction inside of the spinodal region, the decomposition is determined solely by diffusion. Thus, it can be treated purely as a diffusional problem, and many of the characteristics of the decomposition can be described by an approximate analytical solution to the general diffusion equation.In contrast, theories of nucleation and growth have to invoke the thermodynamics of fluctuations. And the diffusional problem involved in the growth of the nucleus is far more difficult to solve, because it is unrealistic to linearize the diffusion equation.From a more practical standpoint, spinodal decomposition provides a means of producing a very finely dispersed microstructure that can significantly enhance the physical properties of the material.








![Keq = [A] [B] [C] [D]](http://s1.studyres.com/store/data/014463360_1-50a2de0db1e8b9a361c4b31c6e85c28d-300x300.png)







 Studied by](http://s1.studyres.com/store/data/014637611_1-0e44dc46987f4ac973f72ef81c275c20-300x300.png)




