Topic 4 - Lloyd Crosby
... The term was first applied to the combining of others elements, particularly metals, with oxygen. e. Reduction A process in which an element gains one or more electrons, causing its oxidation number to decrease The term was first applied to reactions in which oxygen was removed from metal oxides ore ...
... The term was first applied to the combining of others elements, particularly metals, with oxygen. e. Reduction A process in which an element gains one or more electrons, causing its oxidation number to decrease The term was first applied to reactions in which oxygen was removed from metal oxides ore ...
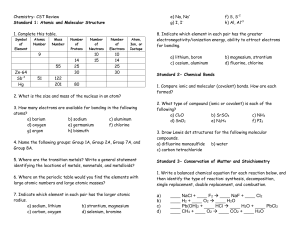
Chemistry- CST Review
... 4. Calculate the molarity of each of the following solutions: a) 0.60 mol of NaCl dissolved in 1.6 L of solution. b) 25.2 g of potassium nitrate, KNO3, in enough water to make 150.0 mL of solution. 5. Calculate the number of grams of solute needed to prepare each of the following solutions: a) 4500. ...
... 4. Calculate the molarity of each of the following solutions: a) 0.60 mol of NaCl dissolved in 1.6 L of solution. b) 25.2 g of potassium nitrate, KNO3, in enough water to make 150.0 mL of solution. 5. Calculate the number of grams of solute needed to prepare each of the following solutions: a) 4500. ...
Section 4.8: Acid-Base Reactions
... Once a solution is standardized, it may be used as a secondary standard for determining the concentration of other solutions with which it reacts. For example, KHP (potassium hydrogen phthalate) is a solid primary standard used to standardize sodium hydroxide (NaOH) solutions. A standardized NaOH so ...
... Once a solution is standardized, it may be used as a secondary standard for determining the concentration of other solutions with which it reacts. For example, KHP (potassium hydrogen phthalate) is a solid primary standard used to standardize sodium hydroxide (NaOH) solutions. A standardized NaOH so ...
5H2O → CuSO4 + 5H2O(g)
... 7. BaCl2(aq) + Na2SO4(aq) BaSO4(s) + 2NaCl(aq) Decomp. 8. 2HgO(s) 2Hg(l) + O2(g) Acid-base neut. 9. 2LiOH(aq) + H2SO4(aq) Li2SO4(aq) + 2H2O(l) Precip. 10. Na2CrO4(aq) + Ni(NO3)2(aq) 2NaNO3(aq) + NiCrO4(s) Combo. 11. 4Li(s) + O2(g) 2Li2O(s) Acid-base neut. 12. Mg(OH)2(aq) + 2HCl(aq) 2MgCl ...
... 7. BaCl2(aq) + Na2SO4(aq) BaSO4(s) + 2NaCl(aq) Decomp. 8. 2HgO(s) 2Hg(l) + O2(g) Acid-base neut. 9. 2LiOH(aq) + H2SO4(aq) Li2SO4(aq) + 2H2O(l) Precip. 10. Na2CrO4(aq) + Ni(NO3)2(aq) 2NaNO3(aq) + NiCrO4(s) Combo. 11. 4Li(s) + O2(g) 2Li2O(s) Acid-base neut. 12. Mg(OH)2(aq) + 2HCl(aq) 2MgCl ...
Objective: The objective of the lab is to study the types of reactions
... You can’t see the salt, but if you let the water evaporate you would get a salt residue in the beaker. The (s) means that the material is not soluble or insoluble. Sand is an insoluble material. If you would put some sand in a beaker of water it would not dissolve. Just putting something in a beaker ...
... You can’t see the salt, but if you let the water evaporate you would get a salt residue in the beaker. The (s) means that the material is not soluble or insoluble. Sand is an insoluble material. If you would put some sand in a beaker of water it would not dissolve. Just putting something in a beaker ...
WRITING AP EQUATIONS AP equation sets are found in the
... Ex. Excess ammonia is added to a solution of zinc nitrate. ...
... Ex. Excess ammonia is added to a solution of zinc nitrate. ...
Introduction
... Acid-base neut. 9. 2LiOH(aq) + H2SO4(aq) Li2SO4(aq) + 2H2O(l) Precip. 10. Na2CrO4(aq) + Ni(NO3)2(aq) 2NaNO3(aq) + NiCrO4(s) Combo. 11. 4Li(s) + O2(g) 2Li2O(s) Acid-base neut. 12. Mg(OH)2(aq) + 2HCl(aq) 2MgCl2(aq) + H2O(l) Combo. 13. NH3(g) + HCl(g) NH4Cl(s) Decomp. 14. NiCO3(s) NiO(s) + ...
... Acid-base neut. 9. 2LiOH(aq) + H2SO4(aq) Li2SO4(aq) + 2H2O(l) Precip. 10. Na2CrO4(aq) + Ni(NO3)2(aq) 2NaNO3(aq) + NiCrO4(s) Combo. 11. 4Li(s) + O2(g) 2Li2O(s) Acid-base neut. 12. Mg(OH)2(aq) + 2HCl(aq) 2MgCl2(aq) + H2O(l) Combo. 13. NH3(g) + HCl(g) NH4Cl(s) Decomp. 14. NiCO3(s) NiO(s) + ...
Example - Request a Spot account
... metal gets oxidized: Na Na+ + e-) b. The nonmetal gains an electron(s) and becomes an anion (reduction nonmetal gets reduced: Cl + e- Cl-) c. In this reaction, electrons are transferred from the metal to the nonmetal 2. O2 as a reactant or product: CH4(s) + O2(g)CO2(g) + H2O(g) a. In this rea ...
... metal gets oxidized: Na Na+ + e-) b. The nonmetal gains an electron(s) and becomes an anion (reduction nonmetal gets reduced: Cl + e- Cl-) c. In this reaction, electrons are transferred from the metal to the nonmetal 2. O2 as a reactant or product: CH4(s) + O2(g)CO2(g) + H2O(g) a. In this rea ...
Chapter 10
... Acids – always aqueous (aq) Ionic compounds – refer to solubility table Soluble (dissolves) – aqueous (aq) Insoluble (doesn’t dissolve) – solid (s) ...
... Acids – always aqueous (aq) Ionic compounds – refer to solubility table Soluble (dissolves) – aqueous (aq) Insoluble (doesn’t dissolve) – solid (s) ...
GEOC: Division of Geochemistry subsurface environment
... 1 Interdisciplinary Sci Engr Lab, Univ of Delaware, Newark, Delaware, United States; 2 Plant and Soil Science, University of Delaware, Hockessin, Delaware, United States Abstract:In order to understand the long-term bioavailability of inorganic pollutants such as chromium(Cr) it is essential to quan ...
... 1 Interdisciplinary Sci Engr Lab, Univ of Delaware, Newark, Delaware, United States; 2 Plant and Soil Science, University of Delaware, Hockessin, Delaware, United States Abstract:In order to understand the long-term bioavailability of inorganic pollutants such as chromium(Cr) it is essential to quan ...
chapter 7 - chemical formulas and chemical compounds
... subscripts showing the smallest whole-number mole ratio of the different atoms in the compound - ionic compounds - formula unit is the compound’s empirical formula - molecular compound - empirical formula does not indicate the actual numbers of atoms present in each molecule - calculate: convert per ...
... subscripts showing the smallest whole-number mole ratio of the different atoms in the compound - ionic compounds - formula unit is the compound’s empirical formula - molecular compound - empirical formula does not indicate the actual numbers of atoms present in each molecule - calculate: convert per ...
Preliminary Course Atomic Structure 1 + 2
... So Calcium would have to react with TWO clorines – Calcium forms CaCl2, not CaCl ...
... So Calcium would have to react with TWO clorines – Calcium forms CaCl2, not CaCl ...
WRITING AP EQUATIONS AP equation sets are found in the free
... Ex. Excess ammonia is added to a solution of zinc nitrate. ...
... Ex. Excess ammonia is added to a solution of zinc nitrate. ...
An element`s properties depend on the structure of its atoms
... attracted to one another, now find each other quite attractive, thus more energy is required to separate them! • In other words, molecules become “sticky” or adhere to one another. • Collectively, such interactions can be strong, as between the molecules of a gecko’s toe hairs and the surface of a w ...
... attracted to one another, now find each other quite attractive, thus more energy is required to separate them! • In other words, molecules become “sticky” or adhere to one another. • Collectively, such interactions can be strong, as between the molecules of a gecko’s toe hairs and the surface of a w ...
Document
... When some metallic hydroxides are heated, they decompose to form metallic oxide and water. Ex: Ca(OH)2 CaO + H2O ...
... When some metallic hydroxides are heated, they decompose to form metallic oxide and water. Ex: Ca(OH)2 CaO + H2O ...
Biologically Important Inorganic Elements Occurrence and Availability
... Transition Metals in Biomolecules Iron. Most abundant metal in biology, used by all plants and animals including bacteria. Some roles duplicated by other metals, while others are unique to Fe. Iron use has survived the evolution of the O2 atmosphere on earth and the instability of Fe(II) with respe ...
... Transition Metals in Biomolecules Iron. Most abundant metal in biology, used by all plants and animals including bacteria. Some roles duplicated by other metals, while others are unique to Fe. Iron use has survived the evolution of the O2 atmosphere on earth and the instability of Fe(II) with respe ...
CHEM1411,chapter 1-2-3 exercises 1. In 1828, the diameter of the
... 5. A piece of a metal alloy with a mass of 114 g was placed into a graduated cylinder that contained 25.0 mL of water, raising the water level to 42.5 mL. What is the density of the metal? A) 0.154 g/cm3 B) 0.592 g/cm3 C) 2.68 g/cm3 D) 6.51 g/cm3 E) 7.25 g/cm3 ...
... 5. A piece of a metal alloy with a mass of 114 g was placed into a graduated cylinder that contained 25.0 mL of water, raising the water level to 42.5 mL. What is the density of the metal? A) 0.154 g/cm3 B) 0.592 g/cm3 C) 2.68 g/cm3 D) 6.51 g/cm3 E) 7.25 g/cm3 ...
Chemical Formulas
... Scientists use chemical formulas such as NaCl instead of common names (table salt) or chemical names (sodium chloride) because it is shorter, more accurate, and universally understood. ...
... Scientists use chemical formulas such as NaCl instead of common names (table salt) or chemical names (sodium chloride) because it is shorter, more accurate, and universally understood. ...
chemical reactions
... 2 H2 + O2 → 2 H2O (premix gases in a pop bottle or ignite a balloon filled with H2 – loud noise) Decomposition Reactions Decomposition is the reverse of combination. That is, a single reactant is broken down into two or more products either elements or compounds. A decomposition reaction will take p ...
... 2 H2 + O2 → 2 H2O (premix gases in a pop bottle or ignite a balloon filled with H2 – loud noise) Decomposition Reactions Decomposition is the reverse of combination. That is, a single reactant is broken down into two or more products either elements or compounds. A decomposition reaction will take p ...
Matter and Energy
... molecules (groups) of the formula -This number will be DISTRIBUTED just like in math. It applies to each element and is multiplied by each subscript to find the total number of atoms of each element and a total number of atoms in the molecule. ...
... molecules (groups) of the formula -This number will be DISTRIBUTED just like in math. It applies to each element and is multiplied by each subscript to find the total number of atoms of each element and a total number of atoms in the molecule. ...
Name: Date: Period: _____ Unit 2 Notes, Part 1 – The Basics of
... (think H-NOF). These bonds are usually depicted with a dotted line. Because they occur between two different molecules and not within one molecule (like ionic or covalent bonds) and they occur between partial (not full) charges, hydrogen bonds are weaker than ionic or covalent bonds. 16. Chemical re ...
... (think H-NOF). These bonds are usually depicted with a dotted line. Because they occur between two different molecules and not within one molecule (like ionic or covalent bonds) and they occur between partial (not full) charges, hydrogen bonds are weaker than ionic or covalent bonds. 16. Chemical re ...
TEK 8.5D: Chemical Formulas
... Scientists use chemical formulas such as NaCl instead of common names (table salt) or chemical names (sodium chloride) because it is shorter, more accurate, and universally understood. ...
... Scientists use chemical formulas such as NaCl instead of common names (table salt) or chemical names (sodium chloride) because it is shorter, more accurate, and universally understood. ...
2. Essential Chemistry
... o Participates in chemical reactions o Water has a high specific heat which moderates temperature - absorbs and releases heat very slowly, minimizes temperature fluctuations to within limits that permit life Heat is absorbed when hydrogen bonds break Heat is released when hydrogen bonds form o R ...
... o Participates in chemical reactions o Water has a high specific heat which moderates temperature - absorbs and releases heat very slowly, minimizes temperature fluctuations to within limits that permit life Heat is absorbed when hydrogen bonds break Heat is released when hydrogen bonds form o R ...
Redox

Redox reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons between species. The term ""redox"" comes from two concepts involved with electron transfer: reduction and oxidation. It can be explained in simple terms: Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion. Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.Although oxidation reactions are commonly associated with the formation of oxides from oxygen molecules, these are only specific examples of a more general concept of reactions involving electron transfer.Redox reactions, or oxidation-reduction reactions, have a number of similarities to acid–base reactions. Like acid–base reactions, redox reactions are a matched set, that is, there cannot be an oxidation reaction without a reduction reaction happening simultaneously. The oxidation alone and the reduction alone are each called a half-reaction, because two half-reactions always occur together to form a whole reaction. When writing half-reactions, the gained or lost electrons are typically included explicitly in order that the half-reaction be balanced with respect to electric charge.Though sufficient for many purposes, these descriptions are not precisely correct. Oxidation and reduction properly refer to a change in oxidation state — the actual transfer of electrons may never occur. The oxidation state of an atom is the fictitious charge that an atom would have if all bonds between atoms of different elements were 100% ionic. Thus, oxidation is better defined as an increase in oxidation state, and reduction as a decrease in oxidation state. In practice, the transfer of electrons will always cause a change in oxidation state, but there are many reactions that are classed as ""redox"" even though no electron transfer occurs (such as those involving covalent bonds).There are simple redox processes, such as the oxidation of carbon to yield carbon dioxide (CO2) or the reduction of carbon by hydrogen to yield methane (CH4), and more complex processes such as the oxidation of glucose (C6H12O6) in the human body through a series of complex electron transfer processes.