Chem 1202 - LSU Department of Chemistry
... The Nature of Energy Potential energy can also be stored • in a battery or a capacitor (electrical potential energy); Each of these forms of energy • in a flywheel can be converted (kinetic potential energy); to other forms by • in the bonds between atoms various processes (chemical potential energy ...
... The Nature of Energy Potential energy can also be stored • in a battery or a capacitor (electrical potential energy); Each of these forms of energy • in a flywheel can be converted (kinetic potential energy); to other forms by • in the bonds between atoms various processes (chemical potential energy ...
CH100: Fundamentals for Chemistry
... To convert from one unit to another: Identify the relationship between the units (e.g. 100 cm = 1 m) Write out the starting measurement and multiply it by a quantity that will yield the desired value: 25 cm ( ...
... To convert from one unit to another: Identify the relationship between the units (e.g. 100 cm = 1 m) Write out the starting measurement and multiply it by a quantity that will yield the desired value: 25 cm ( ...
Solved Examples
... One of the components of acid rain is nitric acid, which forms when NO 2 a pollutant, reacts with oxygen and rainwater according to the following reaction. Assuming that there is more than enough O2 and H2O, how much HNO3 in kilograms forms from 1.5 103 kg of NO2 pollutant? Set up the problem in t ...
... One of the components of acid rain is nitric acid, which forms when NO 2 a pollutant, reacts with oxygen and rainwater according to the following reaction. Assuming that there is more than enough O2 and H2O, how much HNO3 in kilograms forms from 1.5 103 kg of NO2 pollutant? Set up the problem in t ...
File
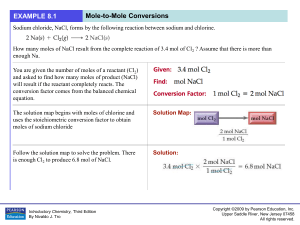
... • The following reaction shows table salt production. How many moles of sodium chloride are produced from 0.02 moles of chlorine? ...
... • The following reaction shows table salt production. How many moles of sodium chloride are produced from 0.02 moles of chlorine? ...
Influence of physical and chemical factors on biological leaching
... Figure 1 shows the dynamics of pH changes during the 21 days of leaching process in biological systems and the control test, carried out with the adjustment of pH to 2,0. Samples inoculated with the smallest quantity of bacteria (10 %, 20 %) required more frequent adjustment of pH, compared to syste ...
... Figure 1 shows the dynamics of pH changes during the 21 days of leaching process in biological systems and the control test, carried out with the adjustment of pH to 2,0. Samples inoculated with the smallest quantity of bacteria (10 %, 20 %) required more frequent adjustment of pH, compared to syste ...
... a wide variety of nucleophilic and electrophilic substitution [1,2], photochemical [3], reduction and oxidation reactions [4,5]. Also, they have been employed as synthons of a wide variety of biologically and medicinally active compounds [6,7], as well as of pharmaceutical compounds having anti-epil ...
Theoretical Competition - Austrian Chemistry Olympiad
... From the above data calculate the potential difference for disproportionation at standard conditions. ΔEƟ = EƟ2 - EƟ3 = 1.763 – 0.695 = 1.068 V In order to avoid spontaneous disproportionation, normally available stabilizers are added to commercial hydrogen peroxide solutions. Additionally, the hydr ...
... From the above data calculate the potential difference for disproportionation at standard conditions. ΔEƟ = EƟ2 - EƟ3 = 1.763 – 0.695 = 1.068 V In order to avoid spontaneous disproportionation, normally available stabilizers are added to commercial hydrogen peroxide solutions. Additionally, the hydr ...
10 4.0 g of magnesium oxide was found to contain 2.4 g of
... Conservation of mass Mass is never lost or gained in chemical reactions. We say that mass is always conserved. In other words, the total mass of products at the end of the reaction is equal to the total mass of the reactants at the beginning. This is because no atoms are created or destroyed during ...
... Conservation of mass Mass is never lost or gained in chemical reactions. We say that mass is always conserved. In other words, the total mass of products at the end of the reaction is equal to the total mass of the reactants at the beginning. This is because no atoms are created or destroyed during ...
2009 Chemistry Midterm Review Packet
... substance. Thermal energy comes from the random motion of the components of the substance. ...
... substance. Thermal energy comes from the random motion of the components of the substance. ...
Example - Schoolwires.net
... a student heats a sample of the hydrate until all the water is gone. A 1.628 g sample of hydrate is heated to constant mass of 1.072 g. What is the value of X? ...
... a student heats a sample of the hydrate until all the water is gone. A 1.628 g sample of hydrate is heated to constant mass of 1.072 g. What is the value of X? ...
PPT - mvhs-fuhsd.org
... proportional to the amount of reactants and products. e.g. for decomposition of two moles of water twice as much energy is needed as for one mole of water. H for a reaction in the forward direction is equal in size, but opposite in sign, to H for the reverse reaction. Reversing a reaction changes ...
... proportional to the amount of reactants and products. e.g. for decomposition of two moles of water twice as much energy is needed as for one mole of water. H for a reaction in the forward direction is equal in size, but opposite in sign, to H for the reverse reaction. Reversing a reaction changes ...
Electrochemistry Lecture
... 1. For an atom in its elemental form (Na, O2, Cl2 …) Ox# = 0 2. For a monatomic ion: Ox# = ion charge 3. The sum of Ox# values for the atoms in a compound equals zero. The sum of Ox# values for the atoms in a polyatomic ion equals the ion charge. Rules for specific atoms or periodic table groups. 1. ...
... 1. For an atom in its elemental form (Na, O2, Cl2 …) Ox# = 0 2. For a monatomic ion: Ox# = ion charge 3. The sum of Ox# values for the atoms in a compound equals zero. The sum of Ox# values for the atoms in a polyatomic ion equals the ion charge. Rules for specific atoms or periodic table groups. 1. ...
Chem 400 Inorganic Chemistry Laboratory
... π electrons. Two molecules of this anion will react with iron(II) to give ferrocene, the most common member of the class of organometallic compounds referred to as metallocenes. In this centrosymmetric sandwich-type π complex, all carbon atoms are equidistant from the iron atom. The extraordinary st ...
... π electrons. Two molecules of this anion will react with iron(II) to give ferrocene, the most common member of the class of organometallic compounds referred to as metallocenes. In this centrosymmetric sandwich-type π complex, all carbon atoms are equidistant from the iron atom. The extraordinary st ...
Unit 8 Packet
... Worksheet 1: Mole Relationships Worksheet 2: Percent Yield Worksheet 3: Adjusting to Reality- Limiting Reactant (3 pages) Worksheet 4- Samples of Every Kind Unit 8 Study Guide ...
... Worksheet 1: Mole Relationships Worksheet 2: Percent Yield Worksheet 3: Adjusting to Reality- Limiting Reactant (3 pages) Worksheet 4- Samples of Every Kind Unit 8 Study Guide ...
Kinetics and Equilibrium
... solids and liquids in calculating Keq. Why? *If temperature is constant, then partial pressure of a gas directly related to the concentration (mol/L) ...
... solids and liquids in calculating Keq. Why? *If temperature is constant, then partial pressure of a gas directly related to the concentration (mol/L) ...
Thermodynamics - WordPress.com
... 31. What is the change in entropy when sugar is dissolved in water? 32. What happens to entropy when water freezes? 33. Give the mathematical form of Gibbs-Helmholtz equation. 34. What is the state of a chemical reaction when i) ∆G = 0 ii) ∆G > 0 iii) ∆G <0 35. Mention the sign of ∆H for the formati ...
... 31. What is the change in entropy when sugar is dissolved in water? 32. What happens to entropy when water freezes? 33. Give the mathematical form of Gibbs-Helmholtz equation. 34. What is the state of a chemical reaction when i) ∆G = 0 ii) ∆G > 0 iii) ∆G <0 35. Mention the sign of ∆H for the formati ...
Sample 112 Final
... Equal masses of two substances, A and B, each absorb 25 Joules of energy. If the temperature of A increases by 4 degrees and the temperature of B increases by 8 degrees, one can say that a) the specific heat of A is double that of B. b) the specific heat of B is double that of A. c) the specific hea ...
... Equal masses of two substances, A and B, each absorb 25 Joules of energy. If the temperature of A increases by 4 degrees and the temperature of B increases by 8 degrees, one can say that a) the specific heat of A is double that of B. b) the specific heat of B is double that of A. c) the specific hea ...
Second Semester Review Part 1
... relation between solubility and temperature? (A) An increase in temperature increases the solubility of a gas in a liquid. (B) The change of solubility with temperature is the same for all substances. (C) The solubility of a liquid in a liquid is independent of temperature. (D) The solubility of mos ...
... relation between solubility and temperature? (A) An increase in temperature increases the solubility of a gas in a liquid. (B) The change of solubility with temperature is the same for all substances. (C) The solubility of a liquid in a liquid is independent of temperature. (D) The solubility of mos ...