PowerPoint Lecture - UCSD Department of Physics
... forward from a stop – the seat you’re sitting in now is exerting an upward force on you (can you feel it?) – you exert a sideways force on a couch that you slide across the floor – a string exerts a centrally-directed (centripetal) force on a rock at the end of a string that you’re twirling over you ...
... forward from a stop – the seat you’re sitting in now is exerting an upward force on you (can you feel it?) – you exert a sideways force on a couch that you slide across the floor – a string exerts a centrally-directed (centripetal) force on a rock at the end of a string that you’re twirling over you ...
Section 1 What Is Energy?
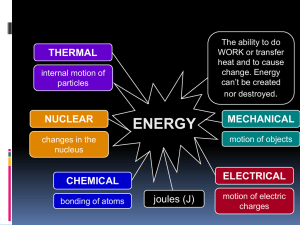
... • Electrical Energy is the energy of moving electrons. Electrical energy can be thought of as potential energy that is used when you plug in an electrical appliance and use it. • Sound Energy is caused by an object’s vibrations. The object’s vibrations transmit some kinetic energy to the air particl ...
... • Electrical Energy is the energy of moving electrons. Electrical energy can be thought of as potential energy that is used when you plug in an electrical appliance and use it. • Sound Energy is caused by an object’s vibrations. The object’s vibrations transmit some kinetic energy to the air particl ...
Document
... 14. How do particles move at higher temperatures compared with how they move at lower temperatures? a. They move slower at higher temperatures. b. They move faster at higher temperatures. c. They move at the same speed at all temperatures. d. They move in circles at higher temperatures. Chemical Ene ...
... 14. How do particles move at higher temperatures compared with how they move at lower temperatures? a. They move slower at higher temperatures. b. They move faster at higher temperatures. c. They move at the same speed at all temperatures. d. They move in circles at higher temperatures. Chemical Ene ...
CH. 9 Sec. 1
... 14. How do particles move at higher temperatures compared with how they move at lower temperatures? a. They move slower at higher temperatures. b. They move faster at higher temperatures. c. They move at the same speed at all temperatures. d. They move in circles at higher temperatures. Chemical Ene ...
... 14. How do particles move at higher temperatures compared with how they move at lower temperatures? a. They move slower at higher temperatures. b. They move faster at higher temperatures. c. They move at the same speed at all temperatures. d. They move in circles at higher temperatures. Chemical Ene ...
8th grade Per.5 Ch5 directed_reading_b
... 14. How do particles move at higher temperatures compared with how they move at lower temperatures? a. They move slower at higher temperatures. b. They move faster at higher temperatures. c. They move at the same speed at all temperatures. d. They move in circles at higher temperatures. Chemical Ene ...
... 14. How do particles move at higher temperatures compared with how they move at lower temperatures? a. They move slower at higher temperatures. b. They move faster at higher temperatures. c. They move at the same speed at all temperatures. d. They move in circles at higher temperatures. Chemical Ene ...
TYPES AND FORMS OF ENERGY
... Potential energy is energy due to position or stored energy. Potential energy is also called gravitational potential energy. ...
... Potential energy is energy due to position or stored energy. Potential energy is also called gravitational potential energy. ...
trt 408 physical chemistry
... arranged in patterns with long range, repeating order. Or Its may be amorphous in which case its atoms or molecules do not have any long range order. ...
... arranged in patterns with long range, repeating order. Or Its may be amorphous in which case its atoms or molecules do not have any long range order. ...
12-1 Chemical Reactions That Involve Heat
... equation would you write the term energy? 2. What is an endothermic reaction? On which side of the chemical equation would you write the term energy? 4. When clouds form water vapor (gas) turns back to liquid water. Is cloud formation exothermic or endothermic? Why do you feel cooler on a hot summer ...
... equation would you write the term energy? 2. What is an endothermic reaction? On which side of the chemical equation would you write the term energy? 4. When clouds form water vapor (gas) turns back to liquid water. Is cloud formation exothermic or endothermic? Why do you feel cooler on a hot summer ...
Course Materials (These materials are for non
... holes in curved surfaces often requires the area to be flattened depending on the local slope of the surface relative to the axis of the drill, drills have very low bending stiffness and strength ...
... holes in curved surfaces often requires the area to be flattened depending on the local slope of the surface relative to the axis of the drill, drills have very low bending stiffness and strength ...
Chapter 0 Introduction to Energy
... of radiant energy. Nuclear energy has the highest storage of energy per gram. That’s why it’s used for some submarines that must go a long time between refueling. Radiant Energy. All the above forms of energy are stored energy—energy waiting to be turned into work, heat, or radiant energy. Radiant e ...
... of radiant energy. Nuclear energy has the highest storage of energy per gram. That’s why it’s used for some submarines that must go a long time between refueling. Radiant Energy. All the above forms of energy are stored energy—energy waiting to be turned into work, heat, or radiant energy. Radiant e ...
Lasers, Yeah They Look Cool, But How Do They Work?
... The Laser Light Process • The process of creating light can be broken down into four stages. The next four slides will show and explain to you how a laser works! ...
... The Laser Light Process • The process of creating light can be broken down into four stages. The next four slides will show and explain to you how a laser works! ...
Randall Snurr Friday, March 4, 2016 10:00-11:00 a.m. 102 Colburn Lab
... structural and chemical properties of the resulting materials can be finely tuned, and this makes MOFs promising materials for applications such as gas storage, chemical separations, sensing, drug delivery, and catalysis. This talk will focus on efforts to design or screen MOFs for separating mixtur ...
... structural and chemical properties of the resulting materials can be finely tuned, and this makes MOFs promising materials for applications such as gas storage, chemical separations, sensing, drug delivery, and catalysis. This talk will focus on efforts to design or screen MOFs for separating mixtur ...
Kinetic Energy
... • Energy is the ability to do work. When work is done, energy is transferred from one object to another. Energy can exist in different forms, such as electrical and chemical energy. Most forms of energy can also be classified as kinetic or potential energy. • Kinetic energy is the energy of moving m ...
... • Energy is the ability to do work. When work is done, energy is transferred from one object to another. Energy can exist in different forms, such as electrical and chemical energy. Most forms of energy can also be classified as kinetic or potential energy. • Kinetic energy is the energy of moving m ...
Module Objective(s) - Students will…
... its arrangement. Please write this down, potential energy stored in a stretched rubber band is called elastic potential energy. Elastic potential energy can be stored in stretched springs as well as stretched rubber bands. In fact, any object that can be forced into a shape that is different from i ...
... its arrangement. Please write this down, potential energy stored in a stretched rubber band is called elastic potential energy. Elastic potential energy can be stored in stretched springs as well as stretched rubber bands. In fact, any object that can be forced into a shape that is different from i ...
Mt. SAC
... • Homogeneous: uniform throughout, appears to be one thing – Pure substances b – Solutions (homogeneous mixtures) ...
... • Homogeneous: uniform throughout, appears to be one thing – Pure substances b – Solutions (homogeneous mixtures) ...
Slide 1 - nanoHUB
... • Often, nanoparticles (nanocrystals) do not form welldefined crystal facets. • The Wulff crystal shapes are idealized cases where the crystal surface energies determine the shape (thermodynamic control). • Kinetic factors often play a major role in crystal growth. This explains why different proces ...
... • Often, nanoparticles (nanocrystals) do not form welldefined crystal facets. • The Wulff crystal shapes are idealized cases where the crystal surface energies determine the shape (thermodynamic control). • Kinetic factors often play a major role in crystal growth. This explains why different proces ...