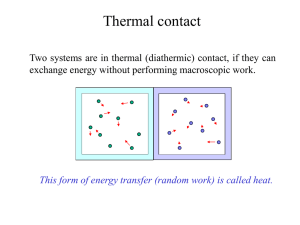
Q - UCSB Physics
... • Thermodynamic system: described by state variables (p, V, T, ..) • Thermodynamic process: changes the state ( p, V, T, ...) of the system • Heat Q, Work W: ‘path-dependent’: values depend on process ...
... • Thermodynamic system: described by state variables (p, V, T, ..) • Thermodynamic process: changes the state ( p, V, T, ...) of the system • Heat Q, Work W: ‘path-dependent’: values depend on process ...
Physics 4230 Set 2 Solutions Fall 1998 Fermi 2.1) Basic 1st Law of
... Here, I just wanted to see decent plots of the PV lines which you had to use in Fermi 2.3 and 2.4. I didn’t necessarily want you to draw isotherms unless you actually used them in the solutions. My major point here is to try to make you internalize the connection between the picture of the PV plane ...
... Here, I just wanted to see decent plots of the PV lines which you had to use in Fermi 2.3 and 2.4. I didn’t necessarily want you to draw isotherms unless you actually used them in the solutions. My major point here is to try to make you internalize the connection between the picture of the PV plane ...
gec221 tutorial kit - Covenant University
... the contributors do not in any way claim authorship or ownership of them. The materials are also not to be used for any commercial purpose. ...
... the contributors do not in any way claim authorship or ownership of them. The materials are also not to be used for any commercial purpose. ...
Mechanical Engineering
... can only talk about the average specific heat, c = Q/mΔT. Since it was customary to give the specific heat as a property in describing a material, methods of analysis came to rely on it for routine calculations. However, since it is only constant for some materials, older calculations became very co ...
... can only talk about the average specific heat, c = Q/mΔT. Since it was customary to give the specific heat as a property in describing a material, methods of analysis came to rely on it for routine calculations. However, since it is only constant for some materials, older calculations became very co ...
Overview
... 6. (a) Evaluate microstates W1 for one particle of a monatomic ideal gas in volume V using the (crude) idea that each particle occupies a cube of volume 3 were the sides are one deBroglie wavelength . The number of ways one particle can fit in V is V/3 and the average energy per particle is U/N=3 ...
... 6. (a) Evaluate microstates W1 for one particle of a monatomic ideal gas in volume V using the (crude) idea that each particle occupies a cube of volume 3 were the sides are one deBroglie wavelength . The number of ways one particle can fit in V is V/3 and the average energy per particle is U/N=3 ...
Physical Chemistry for the Biosciences I (Ch 416 )
... Key to application of these laws is the notion of a system. System is part of universe we choose to focus. There are boundaries between the system and rest of the surroundings. Depending on the nature boundaries we define different types of systems. ...
... Key to application of these laws is the notion of a system. System is part of universe we choose to focus. There are boundaries between the system and rest of the surroundings. Depending on the nature boundaries we define different types of systems. ...
Chapter 14 The Ideal Gas Law and Kinetic Theory
... a) The information given is insufficient to indicate the reason for the increase. b) The increase in internal energy indicates that heat was added to the system. c) The increase in internal energy indicates that work was done by the system. d) The increase in internal energy indicates that heat was ...
... a) The information given is insufficient to indicate the reason for the increase. b) The increase in internal energy indicates that heat was added to the system. c) The increase in internal energy indicates that work was done by the system. d) The increase in internal energy indicates that heat was ...
ph202_overhead_ch15
... where C is the molar heat capacity (J/mol.K) • Since gases it is necessary to distinguish between molar heat capacity at constant pressure (CP) and constant volume (CV) • Let’s begin with the 1st Law of Thermodynamics: Q = DU + W • At constant pressure: {DU = (3/2)nRT & W = PDV = nRDT} Q = (3/2)nRDT ...
... where C is the molar heat capacity (J/mol.K) • Since gases it is necessary to distinguish between molar heat capacity at constant pressure (CP) and constant volume (CV) • Let’s begin with the 1st Law of Thermodynamics: Q = DU + W • At constant pressure: {DU = (3/2)nRT & W = PDV = nRDT} Q = (3/2)nRDT ...
Thermodynamics of ideal gases
... The reason is that the two fundamental laws of thermodynamics are formulated in terms of the energy and the entropy. Both laws concern processes that may take place in an isolated system which is not allowed to exchange heat with or perform work on the environment. The First Law states that the ener ...
... The reason is that the two fundamental laws of thermodynamics are formulated in terms of the energy and the entropy. Both laws concern processes that may take place in an isolated system which is not allowed to exchange heat with or perform work on the environment. The First Law states that the ener ...
Equipartition theorem

In classical statistical mechanics, the equipartition theorem is a general formula that relates the temperature of a system with its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition. The original idea of equipartition was that, in thermal equilibrium, energy is shared equally among all of its various forms; for example, the average kinetic energy per degree of freedom in the translational motion of a molecule should equal that of its rotational motions.The equipartition theorem makes quantitative predictions. Like the virial theorem, it gives the total average kinetic and potential energies for a system at a given temperature, from which the system's heat capacity can be computed. However, equipartition also gives the average values of individual components of the energy, such as the kinetic energy of a particular particle or the potential energy of a single spring. For example, it predicts that every atom in a monatomic ideal gas has an average kinetic energy of (3/2)kBT in thermal equilibrium, where kB is the Boltzmann constant and T is the (thermodynamic) temperature. More generally, it can be applied to any classical system in thermal equilibrium, no matter how complicated. The equipartition theorem can be used to derive the ideal gas law, and the Dulong–Petit law for the specific heat capacities of solids. It can also be used to predict the properties of stars, even white dwarfs and neutron stars, since it holds even when relativistic effects are considered.Although the equipartition theorem makes very accurate predictions in certain conditions, it becomes inaccurate when quantum effects are significant, such as at low temperatures. When the thermal energy kBT is smaller than the quantum energy spacing in a particular degree of freedom, the average energy and heat capacity of this degree of freedom are less than the values predicted by equipartition. Such a degree of freedom is said to be ""frozen out"" when the thermal energy is much smaller than this spacing. For example, the heat capacity of a solid decreases at low temperatures as various types of motion become frozen out, rather than remaining constant as predicted by equipartition. Such decreases in heat capacity were among the first signs to physicists of the 19th century that classical physics was incorrect and that a new, more subtle, scientific model was required. Along with other evidence, equipartition's failure to model black-body radiation—also known as the ultraviolet catastrophe—led Max Planck to suggest that energy in the oscillators in an object, which emit light, were quantized, a revolutionary hypothesis that spurred the development of quantum mechanics and quantum field theory.