Document
... It would seem that the basic idea of the quantum theory is the impossibility of imagining an isolated quantity of energy without associating with it a certain frequency de Broglie in 1923 as a graduate student ...
... It would seem that the basic idea of the quantum theory is the impossibility of imagining an isolated quantity of energy without associating with it a certain frequency de Broglie in 1923 as a graduate student ...
Attosecond lighthouse driven by sub-two
... High-order harmonic generation, the nonlinear interaction between intense laser pulses and an ionizing medium, produces bursts of coherent XUV emission with attosecond pulse duration [1]. These attosecond pulses make the study and control of ultrafast electron dynamics [2] possible up to a time scal ...
... High-order harmonic generation, the nonlinear interaction between intense laser pulses and an ionizing medium, produces bursts of coherent XUV emission with attosecond pulse duration [1]. These attosecond pulses make the study and control of ultrafast electron dynamics [2] possible up to a time scal ...
Frequency Mode Locking in Erbium Doped Fiber Laser Dhyaa . F
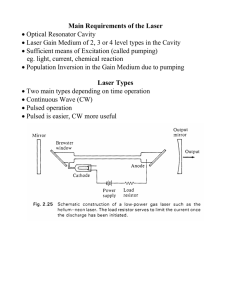
... In the Fabry–Perot laser cavity The light makes two passes through the gain medium per round-trip (RT). Laser system consists of a set of mirrors and a gain medium. The gain medium is an optical amplifier which coherently amplifies light passing through it. The mirrors may be curved or planar and to ...
... In the Fabry–Perot laser cavity The light makes two passes through the gain medium per round-trip (RT). Laser system consists of a set of mirrors and a gain medium. The gain medium is an optical amplifier which coherently amplifies light passing through it. The mirrors may be curved or planar and to ...
Photosynthesis Pt 1 Light
... particular wavelengths of light. • The colors that you see are the wavelengths that are reflected, not absorbed. • If chlorophyll is a green pigment, then what wavelength is not being absorbed? ...
... particular wavelengths of light. • The colors that you see are the wavelengths that are reflected, not absorbed. • If chlorophyll is a green pigment, then what wavelength is not being absorbed? ...
Chapter 7 – Lecture Example Problems 1. A Wavelength of violet
... 3. It requires 280 kJ per mole of Lithium atoms to remove one mole of electrons from the surface of Lithium metal. What minimum wavelength of light is required to do this? ...
... 3. It requires 280 kJ per mole of Lithium atoms to remove one mole of electrons from the surface of Lithium metal. What minimum wavelength of light is required to do this? ...
Fourth lecture, 28.10.03 (dispersion cancellation, time measurement
... Why? No interference between paths leading to different frequencies at the detectors, because in principle one could go back and measure how much energy had been absorbed. Note: it took a long time-integral to enforce this. If the detector had been open only for 1 fs, it would be impossible to tell ...
... Why? No interference between paths leading to different frequencies at the detectors, because in principle one could go back and measure how much energy had been absorbed. Note: it took a long time-integral to enforce this. If the detector had been open only for 1 fs, it would be impossible to tell ...
L 35 Modern Physics [1] - University of Iowa Physics
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
L 35 Modern Physics [1] Modern Physics
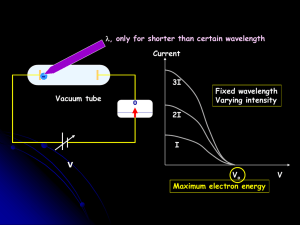
... • A radical idea was needed to explain the photoelectric effect. • Light is an electromagnetic wave, but when it interacts with matter (the metal surface) it behaves like a particle, a light particle called a photon. • A beam of light is thought of as a beam of photons. ...
... • A radical idea was needed to explain the photoelectric effect. • Light is an electromagnetic wave, but when it interacts with matter (the metal surface) it behaves like a particle, a light particle called a photon. • A beam of light is thought of as a beam of photons. ...
L34 - University of Iowa Physics
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
laser
... • With training configuration, 800~900 finesse can be reached constantly. • Also, observed beam waist achieved inside the cavity is stably about 60μm. ...
... • With training configuration, 800~900 finesse can be reached constantly. • Also, observed beam waist achieved inside the cavity is stably about 60μm. ...
laser syllabus 2015 - Colorado School of Mines
... 4. understand how to build and apply a quantitative model of laser oscillation to a real laser system 5. be able to experimentally align and characterize simple lasers Brief list of topics covered: 1. Atom-EM wave interactions: gain and absorption, line broadening 2. Laser pumping, rate equations an ...
... 4. understand how to build and apply a quantitative model of laser oscillation to a real laser system 5. be able to experimentally align and characterize simple lasers Brief list of topics covered: 1. Atom-EM wave interactions: gain and absorption, line broadening 2. Laser pumping, rate equations an ...
Ch. 5 Notes: Electrons in Atoms Big Idea: The Atoms of each
... a. The light of a neon sign is produced by passing electricity through a tube filled with neon gas. Neon atoms in the tube absorb energy and become excited. These excited atoms return to their stable state by emitting light to release that energy. If the light emitted by the neon is passed through a ...
... a. The light of a neon sign is produced by passing electricity through a tube filled with neon gas. Neon atoms in the tube absorb energy and become excited. These excited atoms return to their stable state by emitting light to release that energy. If the light emitted by the neon is passed through a ...









![L 35 Modern Physics [1] - University of Iowa Physics](http://s1.studyres.com/store/data/000679677_1-b925cf8c8f031b0f2b0c09a806312d20-300x300.png)
![L 35 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/001558975_1-84d6e03bc786b63795533f59711ce2f4-300x300.png)