Problem 1: “A brief history” of life in the universe
... atoms escape more readily than nitrogen molecules even though the escape velocity is independent of the mass of the escaping object. The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, inter ...
... atoms escape more readily than nitrogen molecules even though the escape velocity is independent of the mass of the escaping object. The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, inter ...
Problem 1: A brief history of life in the universe
... atoms escape more readily than nitrogen molecules even though the escape velocity is independent of the mass of the escaping object. The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, inter ...
... atoms escape more readily than nitrogen molecules even though the escape velocity is independent of the mass of the escaping object. The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, inter ...
Answers to examination questions
... Q2 A All four molecules are based upon a tetrahedral arrangement of four regions of high electron density. However, lone pairs cause more repulsion than bonding pairs. The oxygen in water is surrounded by two lone pairs (maximum repulsion), the nitrogen atom in ammonia contains one lone pair, bu ...
... Q2 A All four molecules are based upon a tetrahedral arrangement of four regions of high electron density. However, lone pairs cause more repulsion than bonding pairs. The oxygen in water is surrounded by two lone pairs (maximum repulsion), the nitrogen atom in ammonia contains one lone pair, bu ...
Alkanes Chapter 1.1
... Organic Compound • An organic compound is a molecular compound of carbon, not including carbon monoxide, carbon dioxide, and hydrogen cyanide ...
... Organic Compound • An organic compound is a molecular compound of carbon, not including carbon monoxide, carbon dioxide, and hydrogen cyanide ...
Generation of Vapor-Phase Hydrogen Peroxide Test Atmospheres
... –I have a bottle in the bathroom. –How bad can it be? ...
... –I have a bottle in the bathroom. –How bad can it be? ...
Openstax - Chemistry - Answer Key
... 7. Macroscopic. The heat required is determined from macroscopic properties. 9. Liquids can change their shape (flow); solids can’t. Gases can undergo large volume changes as pressure changes; liquids do not. Gases flow and change volume; solids do not. 11. The mixture can have a variety of composit ...
... 7. Macroscopic. The heat required is determined from macroscopic properties. 9. Liquids can change their shape (flow); solids can’t. Gases can undergo large volume changes as pressure changes; liquids do not. Gases flow and change volume; solids do not. 11. The mixture can have a variety of composit ...
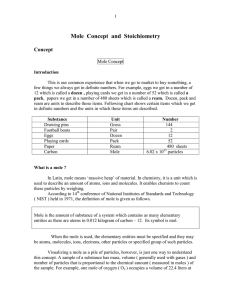
Mole Concept and Stoichiometry
... particles per mole and this value is usually sufficient for solving common problems. Another key point is that the formal definition of a mole does not include a value for Avogadro’s constant and this is probably due to the inherent uncertainty in its measurement. As for the difference between Avoga ...
... particles per mole and this value is usually sufficient for solving common problems. Another key point is that the formal definition of a mole does not include a value for Avogadro’s constant and this is probably due to the inherent uncertainty in its measurement. As for the difference between Avoga ...
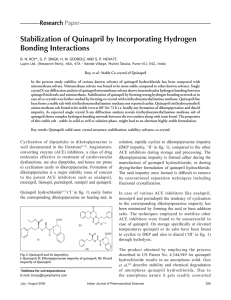
Stabilization of Quinapril by Incorporating Hydrogen Bonding
... and nitromethane. Apparently, acetonitrile solvate of quinapril hydrochloride shows inferior stability to that of nitromethane solvate because no hydrogen bonding has been observed in single crystal X-ray diffraction pattern in the former case. In view of the above, attempts have been made to design ...
... and nitromethane. Apparently, acetonitrile solvate of quinapril hydrochloride shows inferior stability to that of nitromethane solvate because no hydrogen bonding has been observed in single crystal X-ray diffraction pattern in the former case. In view of the above, attempts have been made to design ...
Section 5
... 2. The energy change involved in structural rearrangements 3. Steric contributions 4. Solvent effects ...
... 2. The energy change involved in structural rearrangements 3. Steric contributions 4. Solvent effects ...
First Semester Final Review
... percent. Which of the following is the most likely explanation for this difference? a. Strong initial heating caused some of the hydrate sample to spatter out of the crucible. b. The dehydrated sample absorbed moisture after heating. c. The amount of the hydrate sample used was too small. d. The cru ...
... percent. Which of the following is the most likely explanation for this difference? a. Strong initial heating caused some of the hydrate sample to spatter out of the crucible. b. The dehydrated sample absorbed moisture after heating. c. The amount of the hydrate sample used was too small. d. The cru ...
Answers to examination questions
... All four molecules are based upon a tetrahedral arrangement of four regions of high electron density. However, lone pairs cause more repulsion than bonding pairs. The oxygen in water is surrounded by two lone pairs (maximum repulsion), the nitrogen atom in ammonia contains one lone pair, but the car ...
... All four molecules are based upon a tetrahedral arrangement of four regions of high electron density. However, lone pairs cause more repulsion than bonding pairs. The oxygen in water is surrounded by two lone pairs (maximum repulsion), the nitrogen atom in ammonia contains one lone pair, but the car ...
Chemical Bonding
... charge as far away as possible. In all cases, each ion is surrounded by ions of opposite charge. In theory, this arrangement of ions creates strong attractions. This theory is supported by empirical evidence such as the hard surfaces and high melting and boiling points of ionic solids. ...
... charge as far away as possible. In all cases, each ion is surrounded by ions of opposite charge. In theory, this arrangement of ions creates strong attractions. This theory is supported by empirical evidence such as the hard surfaces and high melting and boiling points of ionic solids. ...
chem A exercise package C
... electron into this overlapping region or into an electron "pool." By doing this, each atom appears to gain an electron within its original boundary. For every overlapping region an atom appears to gain one electron. Two overlapping regions, such as for oxygen, will result in the gain of two electron ...
... electron into this overlapping region or into an electron "pool." By doing this, each atom appears to gain an electron within its original boundary. For every overlapping region an atom appears to gain one electron. Two overlapping regions, such as for oxygen, will result in the gain of two electron ...
Elements, Compounds, and Chemical Equations
... Come to tutoring to use the study guide to find additional facts! ...
... Come to tutoring to use the study guide to find additional facts! ...
Chem 150 Unit 2 - Hydrocarbons & Functional Groups
... Alcohols and Carboxylic acids also have a hydroxyl group with a hydrogen bonded to an oxygen. This allows them to form hydrogen bonds with each other. Therefore, carboxylic acids have at least three different noncovalent interactions: ...
... Alcohols and Carboxylic acids also have a hydroxyl group with a hydrogen bonded to an oxygen. This allows them to form hydrogen bonds with each other. Therefore, carboxylic acids have at least three different noncovalent interactions: ...
Defects and Disorders in Hafnium Oxide and at Hafnium O id /Sili I t f
... are often localized states which can trap electrons or holes and are often termed as trapping centers or simply “traps”; ...
... are often localized states which can trap electrons or holes and are often termed as trapping centers or simply “traps”; ...
Document
... and the closest contact between them is 3.33 Å, a typical distance for the system held by π-π stacking interactions. CH/-π interactions between the ortho hydrogen atom of a pyridine moiety and an sp2 carbon atom of a nearby ring are observed with a mean atomic distance of 2.86 Å. The above character ...
... and the closest contact between them is 3.33 Å, a typical distance for the system held by π-π stacking interactions. CH/-π interactions between the ortho hydrogen atom of a pyridine moiety and an sp2 carbon atom of a nearby ring are observed with a mean atomic distance of 2.86 Å. The above character ...
Energy Matters - Perth Grammar
... From the first two columns of the table, which factor is being kept constant throughout the series of experiments? ...
... From the first two columns of the table, which factor is being kept constant throughout the series of experiments? ...
CfE Advanced Higher Chemistry Unit 2: Organic
... The energy required to promote the electron would be more than offset by the formation of two extra covalent bonds. However, whereas the others would involve 2p orbitals. Spectroscopic measurements show that all four bonds in methane are identical. Let's look at an alkane, ethane for example. Each c ...
... The energy required to promote the electron would be more than offset by the formation of two extra covalent bonds. However, whereas the others would involve 2p orbitals. Spectroscopic measurements show that all four bonds in methane are identical. Let's look at an alkane, ethane for example. Each c ...
Summary - Clydebank High School
... Section (f) – Properties associated with bonding 1. The melting and boiling points of polar substances is ................................... than the melting and boiling points of non-polar substances with similar molecular mass. 2. Ionic and polar substances tend to be ............................ ...
... Section (f) – Properties associated with bonding 1. The melting and boiling points of polar substances is ................................... than the melting and boiling points of non-polar substances with similar molecular mass. 2. Ionic and polar substances tend to be ............................ ...
CHAPtER 9 Properties and reactions of organic compounds
... It is interesting to note that the melting points do not follow the same pattern as the boiling points. In the solid state, the trans isomers can pack more closely than the cis isomers, making the intermolecular forces more effective. cis and trans isomers can also occur in ring structures. cis–tran ...
... It is interesting to note that the melting points do not follow the same pattern as the boiling points. In the solid state, the trans isomers can pack more closely than the cis isomers, making the intermolecular forces more effective. cis and trans isomers can also occur in ring structures. cis–tran ...
Chemistry Packet: Chemical Bonding
... types and numbers of atoms combined in a single molecule a) Ex: hydrogen peroxide; H2O2;________ atoms of hydrogen and _______ atoms of oxygen are held together by covalent bonds b) Ex: glucose; C6H12O6; 6 atoms of carbon, 12 atoms of hydrogen, and 6 atoms of oxygen are held together by ____________ ...
... types and numbers of atoms combined in a single molecule a) Ex: hydrogen peroxide; H2O2;________ atoms of hydrogen and _______ atoms of oxygen are held together by covalent bonds b) Ex: glucose; C6H12O6; 6 atoms of carbon, 12 atoms of hydrogen, and 6 atoms of oxygen are held together by ____________ ...
Grade 11 review answers
... 10) Identify the shape of the following molecules and characterize them as “polar” or “non-polar.” If the molecule is polar, identify the side that would be slightly negative. a) NH3 b) CCl4 c) PF3 d) CO2 e) HCN f) HCF3 g) BH3 h) HI i) O2 j) H2O k) PH3 a) trigonal pyramidal (3 bonds, l lone pair); p ...
... 10) Identify the shape of the following molecules and characterize them as “polar” or “non-polar.” If the molecule is polar, identify the side that would be slightly negative. a) NH3 b) CCl4 c) PF3 d) CO2 e) HCN f) HCF3 g) BH3 h) HI i) O2 j) H2O k) PH3 a) trigonal pyramidal (3 bonds, l lone pair); p ...
Chemistry 400
... 9) A 100.0 mL sample of 0.300 M NaOH is mixed with a 100.0 mL sample of 0.300 M HNO3 in a coffee cup calorimeter. If both solutions were initially at 35.00°C and the temperature of the resulting solution was recorded as 37.00°C, determine the ΔH°rxn (in units of kJ/mol NaOH) for the neutralization r ...
... 9) A 100.0 mL sample of 0.300 M NaOH is mixed with a 100.0 mL sample of 0.300 M HNO3 in a coffee cup calorimeter. If both solutions were initially at 35.00°C and the temperature of the resulting solution was recorded as 37.00°C, determine the ΔH°rxn (in units of kJ/mol NaOH) for the neutralization r ...
the Language of Chemistry
... He was the first person to make the distinction between organic and inorganic compounds. He introduced the classical system of chemical symbols in 1811, in which elements are abbreviated by one or two letters to make a distinct abbreviation from their Latin names. He developed the radical theory of ...
... He was the first person to make the distinction between organic and inorganic compounds. He introduced the classical system of chemical symbols in 1811, in which elements are abbreviated by one or two letters to make a distinct abbreviation from their Latin names. He developed the radical theory of ...
Hydrogen bond
A hydrogen bond is the electrostatic attraction between polar molecules that occurs when a hydrogen (H) atom bound to a highly electronegative atom such as nitrogen (N), oxygen (O) or fluorine (F) experiences attraction to some other nearby highly electronegative atom.These hydrogen-bond attractions can occur between molecules (intermolecular) or within different parts of a single molecule (intramolecular). The hydrogen bond (5 to 30 kJ/mole) is stronger than a van der Waals interaction, but weaker than covalent or ionic bonds. This type of bond can occur in inorganic molecules such as water and in organic molecules like DNA and proteins.Intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °C) compared to the other group 16 hydrides that have no hydrogen bonds. Intramolecular hydrogen bonding is partly responsible for the secondary and tertiary structures of proteins and nucleic acids. It also plays an important role in the structure of polymers, both synthetic and natural.In 2011, an IUPAC Task Group recommended a modern evidence-based definition of hydrogen bonding, which was published in the IUPAC journal Pure and Applied Chemistry. This definition specifies that The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment X–H in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation. An accompanying detailed technical report provides the rationale behind the new definition.