Production of Materials by Jimmy Huang
... The alkene further splits into smaller alkenes until either ethylene or propene (or both) is formed e.g. C5H10 C2H4 + C3H6. Therefore, the overall products of catalytic cracking are alkanes of shorter chain lengths and small alkenes. The catalysts used are inorganic compounds known as zeolites, wh ...
... The alkene further splits into smaller alkenes until either ethylene or propene (or both) is formed e.g. C5H10 C2H4 + C3H6. Therefore, the overall products of catalytic cracking are alkanes of shorter chain lengths and small alkenes. The catalysts used are inorganic compounds known as zeolites, wh ...
Unit 5 Study Guide
... Unit 5 Study Guide: Chemical Reactions 1. What are the 7 diatomic molecules? ...
... Unit 5 Study Guide: Chemical Reactions 1. What are the 7 diatomic molecules? ...
Chemistry PowerPoint
... a. The total mass of the reactants is greater than the total mass of the products b. The total mass of the reactants is less than the total mass of the products c. The total mass of the reactants equals the total mass of the products d. Mass can be created and destroyed ...
... a. The total mass of the reactants is greater than the total mass of the products b. The total mass of the reactants is less than the total mass of the products c. The total mass of the reactants equals the total mass of the products d. Mass can be created and destroyed ...
Document
... (E) More information is needed to answer this question 43. Which of the following is an acid-base neutralization reaction? (A) 2Al(s) + 3H2SO4(aq) Al2(SO4)3(aq) + 3H2(g) (B) SO2(g) + H2O(l) H2SO3(g) (C) LiOH(aq) + HNO3(aq) LiNO3(aq) + H2O(l) (D) 2KBr(aq) + Cl2(g) 2KCl(aq) + Br2(l) (E) CaBr2( ...
... (E) More information is needed to answer this question 43. Which of the following is an acid-base neutralization reaction? (A) 2Al(s) + 3H2SO4(aq) Al2(SO4)3(aq) + 3H2(g) (B) SO2(g) + H2O(l) H2SO3(g) (C) LiOH(aq) + HNO3(aq) LiNO3(aq) + H2O(l) (D) 2KBr(aq) + Cl2(g) 2KCl(aq) + Br2(l) (E) CaBr2( ...
examples of chemical and physical reactions.
... a) Complete these sentences. i. For BURNING to take place, there must be a FUEL, OXYGEN and _________. ii. For RUSTING to take place, there must be IRON, OXYGEN and _________. 3) Many changes take place everyday. Some of them are reversible and others ...
... a) Complete these sentences. i. For BURNING to take place, there must be a FUEL, OXYGEN and _________. ii. For RUSTING to take place, there must be IRON, OXYGEN and _________. 3) Many changes take place everyday. Some of them are reversible and others ...
HYDROGEN PEROXIDE 20%-50%
... Waste Disposal Method: The materials resulting from clean-up operations may be hazardous wastes and, therefore, subject to specific regulations. Package, store, transport, and dispose of all (clean-up) materials and any contaminated ...
... Waste Disposal Method: The materials resulting from clean-up operations may be hazardous wastes and, therefore, subject to specific regulations. Package, store, transport, and dispose of all (clean-up) materials and any contaminated ...
Chem 150 - Fall 2015 Exam I
... Element symbols and names: symbols, names, and spellings are recommended by IUPAC (http://www.iupac.org/). Names are not yet proposed for the elements beyond 111 - those used here are IUPAC’s temporary systematic names (Pure & Appl. Chem., 1979, 51, 381–384). In the USA and some other countries, the ...
... Element symbols and names: symbols, names, and spellings are recommended by IUPAC (http://www.iupac.org/). Names are not yet proposed for the elements beyond 111 - those used here are IUPAC’s temporary systematic names (Pure & Appl. Chem., 1979, 51, 381–384). In the USA and some other countries, the ...
FACTORS AFFECTING PHOTOSYNTHESIS
... 2. Place one shoot into a large test tube containing recently boiled and cooled distilled water. (Keep ...
... 2. Place one shoot into a large test tube containing recently boiled and cooled distilled water. (Keep ...
Chapter 6-student notes
... chemical reaction occurs in which a gas is produced. If the mass of the final mixture is 85g, what mass of gas was produced? ...
... chemical reaction occurs in which a gas is produced. If the mass of the final mixture is 85g, what mass of gas was produced? ...
- International Journal of Multidisciplinary Research and
... natural gas have powered the technology and transportation networks that drive society. This threatens the supply of energy and causes enormous strains to the environment. The international energy agency projects a 33% increase in oil consumption by 2020, which would affect the use and number of aut ...
... natural gas have powered the technology and transportation networks that drive society. This threatens the supply of energy and causes enormous strains to the environment. The international energy agency projects a 33% increase in oil consumption by 2020, which would affect the use and number of aut ...
Chemistry Final Exam Review 2013
... 2H2O2 (aq) 2H2O (l) + O2 Which of the following will increase the rate of the reaction? a. Increasing pressure on the reaction b. Decreasing concentration of the reactants c. Adding a catalyst to the reaction d. Decreasing the temperature of the reaction 66. For a reaction, increasing the temperat ...
... 2H2O2 (aq) 2H2O (l) + O2 Which of the following will increase the rate of the reaction? a. Increasing pressure on the reaction b. Decreasing concentration of the reactants c. Adding a catalyst to the reaction d. Decreasing the temperature of the reaction 66. For a reaction, increasing the temperat ...
Organic compounds are covalent compounds composed of carbon
... composed of carbonbased molecules. More than 90% of all compounds belong to this group. ...
... composed of carbonbased molecules. More than 90% of all compounds belong to this group. ...
2009-10 Chemistry 1st Semester Final Exam Topics and Review
... d. Hydrogen gas reacts with nitrogen gas to form ammonia. Write and balance a chemical equation for this reaction. e. One of the problems with space travel is the building up of carbon dioxide produced by the astronauts. The typical procedure is to react the carbon dioxide with lithium hydroxide to ...
... d. Hydrogen gas reacts with nitrogen gas to form ammonia. Write and balance a chemical equation for this reaction. e. One of the problems with space travel is the building up of carbon dioxide produced by the astronauts. The typical procedure is to react the carbon dioxide with lithium hydroxide to ...
CELSA - Collaborative research project - Application form
... Project title: Concerto for solids and biocatalysts - Cascade biocatalysis and heterogeneous catalysis for fine chemicals production (ConSolid) Summary: Complex pharmaceuticals often have the desired medical effect only in one ‘enantiomeric’ form, while the mirror image may have no or even adverse e ...
... Project title: Concerto for solids and biocatalysts - Cascade biocatalysis and heterogeneous catalysis for fine chemicals production (ConSolid) Summary: Complex pharmaceuticals often have the desired medical effect only in one ‘enantiomeric’ form, while the mirror image may have no or even adverse e ...
C1a - Mr Corfe
... to the left of the periodic table or further down in the group (not including groups 3-8) TYPES OF REACTIONS PHYSICAL – changing of states EXOTHERMIC – gives out heat ENDOTHERMIC – take in heat from it surrounding THERMAL DECOMPOSITION – is a chemical reaction where a single compound breaks up into ...
... to the left of the periodic table or further down in the group (not including groups 3-8) TYPES OF REACTIONS PHYSICAL – changing of states EXOTHERMIC – gives out heat ENDOTHERMIC – take in heat from it surrounding THERMAL DECOMPOSITION – is a chemical reaction where a single compound breaks up into ...
Title - Iowa State University
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
CHEMICAL REACTION
... meat in a pot, drain fat, add chopped onion. Open the beans and drain them. Open the tomatoes. Add tomatoes, beans, chili powder, salt and pepper to the beef. Heat to boiling. Simmer with a lid on for 1 hour. This recipe makes enough chili for 6 servings of chili. • Ingredients, form, amount, condit ...
... meat in a pot, drain fat, add chopped onion. Open the beans and drain them. Open the tomatoes. Add tomatoes, beans, chili powder, salt and pepper to the beef. Heat to boiling. Simmer with a lid on for 1 hour. This recipe makes enough chili for 6 servings of chili. • Ingredients, form, amount, condit ...
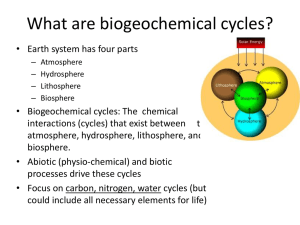
What are biogeochemical cycles?
... • Biogeochemical cycles: The chemical interactions (cycles) that exist between the atmosphere, hydrosphere, lithosphere, and biosphere. • Abiotic (physio-chemical) and biotic processes drive these cycles • Focus on carbon, nitrogen, water cycles (but could include all necessary elements for life) ...
... • Biogeochemical cycles: The chemical interactions (cycles) that exist between the atmosphere, hydrosphere, lithosphere, and biosphere. • Abiotic (physio-chemical) and biotic processes drive these cycles • Focus on carbon, nitrogen, water cycles (but could include all necessary elements for life) ...
Answers for Review Questions Exam 3
... 9. Electrolysis is the use of an electric current to bring about a chemical change. Reduction and oxidation both occur at the same place as in a galvanic cell, but they have different polarities, - and + respectively. It differs from a galvanic cell in that it is in the opposite direction of a galva ...
... 9. Electrolysis is the use of an electric current to bring about a chemical change. Reduction and oxidation both occur at the same place as in a galvanic cell, but they have different polarities, - and + respectively. It differs from a galvanic cell in that it is in the opposite direction of a galva ...
Answers for Review Questions Exam 3
... 10. Electrolysis is used as a source of elements from their ions. Ex. Na from Molten NaCl, Cl2 from a NaCl solution. 11. 0.1663 A current is needed. 12. First 2.47 Volts should be 2.47 Amperes. That gives 4.100g of Fe deposited. 13. Corrosion is the loss of metals to a solution of some form. The pro ...
... 10. Electrolysis is used as a source of elements from their ions. Ex. Na from Molten NaCl, Cl2 from a NaCl solution. 11. 0.1663 A current is needed. 12. First 2.47 Volts should be 2.47 Amperes. That gives 4.100g of Fe deposited. 13. Corrosion is the loss of metals to a solution of some form. The pro ...
UN1001: Section 11: Hydrogen Effects
... Hydride-forming metals are susceptible to H- embrittlement . . .e.g., Zr-alloy pressure tubes (in CANDUs) and fuel sheathing (in all water- cooled reactors) pick up hydrogen (or deuterium in heavy water ) by general corrosion. The hydrogen (D) migrates through the metal lattice to cool regions and t ...
... Hydride-forming metals are susceptible to H- embrittlement . . .e.g., Zr-alloy pressure tubes (in CANDUs) and fuel sheathing (in all water- cooled reactors) pick up hydrogen (or deuterium in heavy water ) by general corrosion. The hydrogen (D) migrates through the metal lattice to cool regions and t ...
Chapter 13 Spectroscopy NMR, IR, MS, UV-Vis
... Since nuclei produce magnetic fields (the ones we’ve been talking about aligning with and against the field), those fields would affect the effective field felt by the hydrogen being measured. In the high energy state they would oppose (reduce) the field and in the low energy state reinforce (increa ...
... Since nuclei produce magnetic fields (the ones we’ve been talking about aligning with and against the field), those fields would affect the effective field felt by the hydrogen being measured. In the high energy state they would oppose (reduce) the field and in the low energy state reinforce (increa ...
Chemistry Study Guide
... 6. What kind of bond is NaCl? Ionic CO2 Covalent N2 Covalent 7. Which group forms acids with H+ ion? Halogens (Group 17) 8. How many valence electrons are in a Group 1 element? 1 Group 13? 3 9. How do positive and negative ions form? Positive ions form when an atom loses an electron, negative ions f ...
... 6. What kind of bond is NaCl? Ionic CO2 Covalent N2 Covalent 7. Which group forms acids with H+ ion? Halogens (Group 17) 8. How many valence electrons are in a Group 1 element? 1 Group 13? 3 9. How do positive and negative ions form? Positive ions form when an atom loses an electron, negative ions f ...
Biology\Ch 2 Chemistry
... A mixture - 2 or more elements or compounds physically mixed together but not chemically combined. Ex: salt mixed with sand When something like NaCl (salt) is mixed in water and the salt breaks into Na+ and Cl- ions, they equally disperse. This is a solution. The salt and water do not recombine to m ...
... A mixture - 2 or more elements or compounds physically mixed together but not chemically combined. Ex: salt mixed with sand When something like NaCl (salt) is mixed in water and the salt breaks into Na+ and Cl- ions, they equally disperse. This is a solution. The salt and water do not recombine to m ...
Artificial photosynthesis

Artificial photosynthesis is a chemical process that replicates the natural process of photosynthesis, a process that converts sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel (a solar fuel). Photocatalytic water splitting converts water into Hydrogen Ions and oxygen, and is a main research area in artificial photosynthesis. Light-driven carbon dioxide reduction is another studied process, replicating natural carbon fixation.Research developed in this field encompasses design and assembly of devices (and their components) for the direct production of solar fuels, photoelectrochemistry and its application in fuel cells, and engineering of enzymes and photoautotrophic microorganisms for microbial biofuel and biohydrogen production from sunlight. Many, if not most, of the artificial approaches are bio-inspired, i.e., they rely on biomimetics.