The placement of an element on the periodic table gives clues about
... The periodic table is so named because it is organized into "periods". A period is defined as an interval required for a cycle to repeat itself. In the periodic table, the periods are the horizontal rows that extend from left to right. These periods consist of as few as two elements and as many as t ...
... The periodic table is so named because it is organized into "periods". A period is defined as an interval required for a cycle to repeat itself. In the periodic table, the periods are the horizontal rows that extend from left to right. These periods consist of as few as two elements and as many as t ...
Chapter 6 review
... 21. Which family is composed of elements that are gases and unreactive? _____________________________ 22. Which family of elements includes soft, reactive metals that form an ion with a +1 charge?____________ 23. Which family of elements includes inert (nonreactive) gases that glow when energized wi ...
... 21. Which family is composed of elements that are gases and unreactive? _____________________________ 22. Which family of elements includes soft, reactive metals that form an ion with a +1 charge?____________ 23. Which family of elements includes inert (nonreactive) gases that glow when energized wi ...
of the periodic table
... 63 known elements Each contained element's symbol, atomic weight and characteristic chemical and physical properties Arranged the cards in order of ascending atomic weight. Elements fell into vertical groups of elements of similar properties ...
... 63 known elements Each contained element's symbol, atomic weight and characteristic chemical and physical properties Arranged the cards in order of ascending atomic weight. Elements fell into vertical groups of elements of similar properties ...
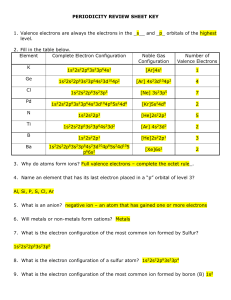
periodicity review sheet key
... Using the graphs from your notes, period table or text book, answer the following with: increases(I), decreases (D), or remains the same (R). __R___ 14. As the number of neutrons in an atom of a given element increases, its atomic number generally ________________ . __I___ 15. Going down a group of ...
... Using the graphs from your notes, period table or text book, answer the following with: increases(I), decreases (D), or remains the same (R). __R___ 14. As the number of neutrons in an atom of a given element increases, its atomic number generally ________________ . __I___ 15. Going down a group of ...
Periodic Table of Elements
... electrons in their outer energy level. • This means that they tend to accept electrons when they combine chemically. • Those with 8 valence electrons are stable and non-reactive. ...
... electrons in their outer energy level. • This means that they tend to accept electrons when they combine chemically. • Those with 8 valence electrons are stable and non-reactive. ...
Ch. 6 The Periodic Table and Periodic Law Vocabulary Review
... ______________________proposed an arrangement where elements were ordered by increasing atomic mass. Newlands noticed when the elements were arranged by increasing atomic mass, their properties repeated every eighth element. Meyer and Mendeleev both demonstrated a connection between ________________ ...
... ______________________proposed an arrangement where elements were ordered by increasing atomic mass. Newlands noticed when the elements were arranged by increasing atomic mass, their properties repeated every eighth element. Meyer and Mendeleev both demonstrated a connection between ________________ ...
module-21 (worksheet-1)
... (b) two elements that have two electrons in their outermost shells.______________________. (c) three elements with filled outermost shells._____________________________________. 3) (a) Lithium, sodium, potassium are all metals that react with water to liberate hydrogen gas. Is there any similarity i ...
... (b) two elements that have two electrons in their outermost shells.______________________. (c) three elements with filled outermost shells._____________________________________. 3) (a) Lithium, sodium, potassium are all metals that react with water to liberate hydrogen gas. Is there any similarity i ...
Objective - davis.k12.ut.us
... a. Dmitri Mendeleev created the first periodic table in 1869. He discovered that the elements shared certain patterns and trends. He observed that the elements shared some chemical and physical properties. Dmitri believed the properties that the elements shared were important. Therefore, he wrote do ...
... a. Dmitri Mendeleev created the first periodic table in 1869. He discovered that the elements shared certain patterns and trends. He observed that the elements shared some chemical and physical properties. Dmitri believed the properties that the elements shared were important. Therefore, he wrote do ...
The Periodic Table Notes
... elements. IV. The Periodic Table a. Dmitri Mendeleev created the first periodic table in 1869. He discovered that the elements shared certain patterns and trends. He observed that the elements shared some chemical and physical properties. Dmitri believed the properties that the elements shared were ...
... elements. IV. The Periodic Table a. Dmitri Mendeleev created the first periodic table in 1869. He discovered that the elements shared certain patterns and trends. He observed that the elements shared some chemical and physical properties. Dmitri believed the properties that the elements shared were ...
How to Read the Periodic Table
... Periodic Table Courtesy of Periodic Table of the Elements v. 4.0 by Kostas Tsigaridis (http://ptoe.move.to/) ...
... Periodic Table Courtesy of Periodic Table of the Elements v. 4.0 by Kostas Tsigaridis (http://ptoe.move.to/) ...
Periodic Table Funsheet
... 9. Where is the highest electronegativity found? _________________________________________________ 10. Where is the lowest electronegativity found? __________________________________________________ 11. Elements of Group 1 are called _____________________________________________________________. 12. ...
... 9. Where is the highest electronegativity found? _________________________________________________ 10. Where is the lowest electronegativity found? __________________________________________________ 11. Elements of Group 1 are called _____________________________________________________________. 12. ...
The Periodic Table
... He studied the patterns of the elements. He was able to organize the elements at the time in order of their atomic mass. He realized that the physical and chemical properties of elements were related to their atomic mass in a ‘periodic’ way. He arranged them so that groups of elements with similar p ...
... He studied the patterns of the elements. He was able to organize the elements at the time in order of their atomic mass. He realized that the physical and chemical properties of elements were related to their atomic mass in a ‘periodic’ way. He arranged them so that groups of elements with similar p ...
In modern periodic table, elements in the same column have similar
... Mendeleev's table • In order to put some elements in the right column, gaps had to be left in his table. • He predicted elements would be discovered to fill the gaps • Also correctly predicted properties of these undiscovered elements ...
... Mendeleev's table • In order to put some elements in the right column, gaps had to be left in his table. • He predicted elements would be discovered to fill the gaps • Also correctly predicted properties of these undiscovered elements ...
Historical development of the nature of matter Democritus (~460
... ♦ For transition metals it is more complicated (not discussed until Chem 422) Octet Rule⇒ atoms tend to ionize or combine such that they achieve eight valence electrons ♦ This would be a electronic configuration the same as the nearest noble gas ♦ Ions with charges in excess of ± 3 are unstable and ...
... ♦ For transition metals it is more complicated (not discussed until Chem 422) Octet Rule⇒ atoms tend to ionize or combine such that they achieve eight valence electrons ♦ This would be a electronic configuration the same as the nearest noble gas ♦ Ions with charges in excess of ± 3 are unstable and ...
File
... except for Period 1. Across a period from left to right, the elements become ___________________ and ___________________ in their properties. o Most ____________________________________ are on the left side of the table (group 1) o Most ____________________________________ are on the right side (gro ...
... except for Period 1. Across a period from left to right, the elements become ___________________ and ___________________ in their properties. o Most ____________________________________ are on the left side of the table (group 1) o Most ____________________________________ are on the right side (gro ...
File
... a. The tendency to lose electrons from the outermost shell of an atom is called the metallic character of an element. b. The metallic character decreases across a period and increases down the group. c. The tendency to gain electrons in the outermost shell of an atom is called the non-metallic chara ...
... a. The tendency to lose electrons from the outermost shell of an atom is called the metallic character of an element. b. The metallic character decreases across a period and increases down the group. c. The tendency to gain electrons in the outermost shell of an atom is called the non-metallic chara ...
Chemical Periodicity - Fort Thomas Independent Schools
... generally increases as you move from left to right across a period. The atomic number and therefore positive charge increases and the shielding effect is constant as you move across. A greater attraction of the nucleus for the electron leads to the increase in ...
... generally increases as you move from left to right across a period. The atomic number and therefore positive charge increases and the shielding effect is constant as you move across. A greater attraction of the nucleus for the electron leads to the increase in ...
6-1-Periodic Law
... It was found that if Mendeleev's table was ordered by atomic number instead of atomic mass the inconsistencies in the table were eliminated. This is the blueprint for the modern periodic table. ...
... It was found that if Mendeleev's table was ordered by atomic number instead of atomic mass the inconsistencies in the table were eliminated. This is the blueprint for the modern periodic table. ...
Chemical Periodicity - Fort Thomas Independent Schools
... generally increases as you move from left to right across a period. The atomic number and therefore positive charge increases and the shielding effect is constant as you move across. A greater attraction of the nucleus for the electron leads to the increase in ...
... generally increases as you move from left to right across a period. The atomic number and therefore positive charge increases and the shielding effect is constant as you move across. A greater attraction of the nucleus for the electron leads to the increase in ...
Elements and the Periodic Table
... • The main body of the periodic table is arranged into 18 vertical columns and seven horizontal rows. • The elements in a column are called a group. Groups are also known as families. • Notice that each group is numbered, from Group 1 on the left of the table to Group 18 on the right. • The group is ...
... • The main body of the periodic table is arranged into 18 vertical columns and seven horizontal rows. • The elements in a column are called a group. Groups are also known as families. • Notice that each group is numbered, from Group 1 on the left of the table to Group 18 on the right. • The group is ...
Anticipation Guide Before After The periodic table is a collection of
... The atomic mass is the sum of protons and electrons in the nucleus. The atomic mass is written above the element on the Periodic Table. Atomic mass can be located mostly in the nucleus (the most dense part of the atom). The majority of the elements on the periodic table are metals. Metals are locate ...
... The atomic mass is the sum of protons and electrons in the nucleus. The atomic mass is written above the element on the Periodic Table. Atomic mass can be located mostly in the nucleus (the most dense part of the atom). The majority of the elements on the periodic table are metals. Metals are locate ...
Periodic table of elements
... All atoms (except hydrogen) want to have 8 electrons in their very outermost energy level (This is called the rule of octet.) Atoms bond until this level is complete. Atoms with few valence electrons lose them during bonding. Atoms with 6, 7, or 8 valence electrons gain electrons during bonding. ...
... All atoms (except hydrogen) want to have 8 electrons in their very outermost energy level (This is called the rule of octet.) Atoms bond until this level is complete. Atoms with few valence electrons lose them during bonding. Atoms with 6, 7, or 8 valence electrons gain electrons during bonding. ...
Unit 3, Lesson 2 Template
... Recall that elements from the same Group will have similar qualities, such as their basic classification. Today we will see that there are other trends that run throughout the Table and once again, elements from the same Group have similar: Atomic Radii sizes, Ionization Energies, and Electron Affin ...
... Recall that elements from the same Group will have similar qualities, such as their basic classification. Today we will see that there are other trends that run throughout the Table and once again, elements from the same Group have similar: Atomic Radii sizes, Ionization Energies, and Electron Affin ...