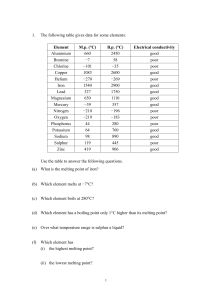
1 - PetyaPisanScienceAQ
... Record both observations: 3) The observations from step one will help you narrow your choice of possible equations for the reaction. To determine which of the remaining possible reactions is the correct one you must obtain quantitative data. You will be completing the table below: (2 marks) Mass of ...
... Record both observations: 3) The observations from step one will help you narrow your choice of possible equations for the reaction. To determine which of the remaining possible reactions is the correct one you must obtain quantitative data. You will be completing the table below: (2 marks) Mass of ...
Sodium - Alberta Health Services
... Copyright © (2013) Alberta Health Services. All rights reserved. These materials may not be changed without written permission from [email protected]. These are intended for general information only; they are provided on an "as is", "where is" basis and are not meant to rep ...
... Copyright © (2013) Alberta Health Services. All rights reserved. These materials may not be changed without written permission from [email protected]. These are intended for general information only; they are provided on an "as is", "where is" basis and are not meant to rep ...
Avoiding Errors in the Reconstitution of Dantrolene Sodium
... Upon the introduction of 60 mL of sterile normal saline into DS‐IV vials, a cloudy (milky) suspension is formed indicating the formation of insoluble particles (Figure, vial A). Centrifugation with subsequent filtration of the particles resulted in their isolation. The separated precipitate was ...
... Upon the introduction of 60 mL of sterile normal saline into DS‐IV vials, a cloudy (milky) suspension is formed indicating the formation of insoluble particles (Figure, vial A). Centrifugation with subsequent filtration of the particles resulted in their isolation. The separated precipitate was ...
TRICYCLIC ANTIDEPRESSANTS
... membrane bicarbonate exchanger in NaHCO3 therapy of imipramine cardiac dysfunction J Toxicol Clin Toxicol ...
... membrane bicarbonate exchanger in NaHCO3 therapy of imipramine cardiac dysfunction J Toxicol Clin Toxicol ...
SODIUM ACETATE
... mild metabolic alkalosis and increased energy expenditure to a similar magnitude. However, sodium bicarbonate ingestion increased fat oxidation but sodium acetate did not, despite the fact that both sodium salts induced a similar increase in energy expenditure and shift in acid-base balance. The aut ...
... mild metabolic alkalosis and increased energy expenditure to a similar magnitude. However, sodium bicarbonate ingestion increased fat oxidation but sodium acetate did not, despite the fact that both sodium salts induced a similar increase in energy expenditure and shift in acid-base balance. The aut ...
Fluid and Electrolytes All Slides
... Monitor Fluid Gains & Losses, restrict sodium, give water Monitor Changes in Behavior Institute Safety Precautions ***Provide tap water to tube fed clients*** ...
... Monitor Fluid Gains & Losses, restrict sodium, give water Monitor Changes in Behavior Institute Safety Precautions ***Provide tap water to tube fed clients*** ...
Class 9 CBSE Test paper Solved Chapter 3: Atoms and...
... 1) Dalton’s atomic theory provided an explanation for the law of conservation of mass and the law of definite proportions. 2) According to Dalton’s atomic theory, all matter (whether an element, a compound or a mixture), is composed of small particles, called atoms. 11.Q. Which of the following pair ...
... 1) Dalton’s atomic theory provided an explanation for the law of conservation of mass and the law of definite proportions. 2) According to Dalton’s atomic theory, all matter (whether an element, a compound or a mixture), is composed of small particles, called atoms. 11.Q. Which of the following pair ...
nomenclature review
... _______ tarnishes rapidly in air _______ boiling point of 883 C _______ soft, silver-white _______ reacts violently with water _______ reacts with acid ...
... _______ tarnishes rapidly in air _______ boiling point of 883 C _______ soft, silver-white _______ reacts violently with water _______ reacts with acid ...
IODOTOPE®
... Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides. As in the use o ...
... Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides. As in the use o ...
Sodium and Salt
... To help readers cut the sodium, she developed her own rendition of a similar blueberry muffin with 87 mg sodium. Health Canada recommends a daily upper intake of 2,300 mg of sodium for an average Canadian adult, which is about 5 ml (1 tsp), and encourages Canadians to reduce that to close to 1,500 ...
... To help readers cut the sodium, she developed her own rendition of a similar blueberry muffin with 87 mg sodium. Health Canada recommends a daily upper intake of 2,300 mg of sodium for an average Canadian adult, which is about 5 ml (1 tsp), and encourages Canadians to reduce that to close to 1,500 ...
General
... Conclusions of these studies are that solubility and stability in both hard and soft water is very good. Solubility is no problem: in this study it was shown that up to 100g/L is dissolved in a few seconds. 24 hours after dissolution we still find 100% of the compound back when analysed. ...
... Conclusions of these studies are that solubility and stability in both hard and soft water is very good. Solubility is no problem: in this study it was shown that up to 100g/L is dissolved in a few seconds. 24 hours after dissolution we still find 100% of the compound back when analysed. ...
File - Garbally Chemistry
... Stable-does not react with the gases in the air and has good solubility. High relative molecular mass of Ammonium Iron (II) Sulphate(392) ensures a high degree of accuracy when weighing. When dissolving in water, sulphuric acid must be added to prevent it from reacting with the water (HYDROLYSIS) an ...
... Stable-does not react with the gases in the air and has good solubility. High relative molecular mass of Ammonium Iron (II) Sulphate(392) ensures a high degree of accuracy when weighing. When dissolving in water, sulphuric acid must be added to prevent it from reacting with the water (HYDROLYSIS) an ...
Following a 2 gram sodium diet
... abbreviated as 2,000 mg.) a day. Just one teaspoon of salt contains 2,300 mg. of sodium. ...
... abbreviated as 2,000 mg.) a day. Just one teaspoon of salt contains 2,300 mg. of sodium. ...
Answers
... chemical properties, as they only have the same number of outermost shell electrons, not the same number of electrons. [1] (e) Sodium melted into a silvery ball [1] which moved about very quickly on the water surface, [1] producing a hissing sound [1] before finally disappearing completely [1]. (f) ...
... chemical properties, as they only have the same number of outermost shell electrons, not the same number of electrons. [1] (e) Sodium melted into a silvery ball [1] which moved about very quickly on the water surface, [1] producing a hissing sound [1] before finally disappearing completely [1]. (f) ...
Slide 1 - Dartmouth
... High Blood Pressure Impairs… • Vision and can cause Blindness – blood vessels in eyes rupture or bleed when blood pressure is high ...
... High Blood Pressure Impairs… • Vision and can cause Blindness – blood vessels in eyes rupture or bleed when blood pressure is high ...
I CAN write Chemical formulas
... 1. Write the oxidation number above each (putting parantheses around the polyatomic ion). 2. Cross these and write the oxidation number (without plus or minus charge) of each ion as the subscript of the other atom/polyatomic ion. ...
... 1. Write the oxidation number above each (putting parantheses around the polyatomic ion). 2. Cross these and write the oxidation number (without plus or minus charge) of each ion as the subscript of the other atom/polyatomic ion. ...
Hyvisc - Boehringer Ingelheim Vetmedica
... as a single 2 mL dose. Chemically, hyaluronic acid is a high molecular weight mucopolysaccharide composed of repeating disaccharide units, each unit consisting of D-glucuronic acid and N-acetyl-D-glucosamine. Each mL of Hyvisc Injection contains 11 mg of hyaluronate sodium and 8.47 mg of sodium chlo ...
... as a single 2 mL dose. Chemically, hyaluronic acid is a high molecular weight mucopolysaccharide composed of repeating disaccharide units, each unit consisting of D-glucuronic acid and N-acetyl-D-glucosamine. Each mL of Hyvisc Injection contains 11 mg of hyaluronate sodium and 8.47 mg of sodium chlo ...
Dear Notetaker:
... Ascorbate then moves down its concentration gradient out of cell probably o Not 100% positive on mechanism of this Water is following all the ions out into the aqueous o Important pumps for maintaining pH and bicarb Take advantage of metabolism occurring in the cells Have a balance of charge ...
... Ascorbate then moves down its concentration gradient out of cell probably o Not 100% positive on mechanism of this Water is following all the ions out into the aqueous o Important pumps for maintaining pH and bicarb Take advantage of metabolism occurring in the cells Have a balance of charge ...
Kionex - Perrigo
... (approximately 4 level teaspoons) of Kionex® one to four times daily. One gram of Kionex® contains 4.1 mEq of sodium; one level teaspoon contains approximately 3.5 g of Kionex® and 15 mEq of sodium (A heaping teaspoon may contain as much as 10 g to 12 g of Kionex®). Since the in vivo efficiency of s ...
... (approximately 4 level teaspoons) of Kionex® one to four times daily. One gram of Kionex® contains 4.1 mEq of sodium; one level teaspoon contains approximately 3.5 g of Kionex® and 15 mEq of sodium (A heaping teaspoon may contain as much as 10 g to 12 g of Kionex®). Since the in vivo efficiency of s ...
Chapter 6 Notes - Discount Flies
... Evidence for a chemical reaction is sometimes visual, and can include any or all of the following: 1) Color Change 2) Solid Forms 3) Gas Given Off 4) Heat, Cold or Flames ...
... Evidence for a chemical reaction is sometimes visual, and can include any or all of the following: 1) Color Change 2) Solid Forms 3) Gas Given Off 4) Heat, Cold or Flames ...
Part IB Summary of Product Characteristics
... Symptomatic treatment of moderate to very severe gastro-oesophageal reflux disease (symptomatic GORD): 10 mg once daily in patients without oesophagitis. If symptom control has not been achieved during four weeks, the patient should be further investigated. Once symptoms have resolved, subsequent sy ...
... Symptomatic treatment of moderate to very severe gastro-oesophageal reflux disease (symptomatic GORD): 10 mg once daily in patients without oesophagitis. If symptom control has not been achieved during four weeks, the patient should be further investigated. Once symptoms have resolved, subsequent sy ...
5 Tips for Creating Flavorful Low
... cells that transmit information to muscles and nerves. It also helps your small intestine absorb nutrients. However, too much sodium in your diet can lead to high blood pressure, which can play a part in heart disease, kidney disease or strokes. So, even though you need sodium, you're on the right t ...
... cells that transmit information to muscles and nerves. It also helps your small intestine absorb nutrients. However, too much sodium in your diet can lead to high blood pressure, which can play a part in heart disease, kidney disease or strokes. So, even though you need sodium, you're on the right t ...
FOOD PRESERVATIVES
... • Sorbicfamily : Potassium Sorbate, Sodium Sorbate, Calcium Sorbate • Potasium Sorbate will produce Sorbic Acid once its dissolves in water • Widely used preservative in the world • Effective against yeast, molds, and bacteria • Effective up to pH 6.5 • Maximum level allowable by law is ...
... • Sorbicfamily : Potassium Sorbate, Sodium Sorbate, Calcium Sorbate • Potasium Sorbate will produce Sorbic Acid once its dissolves in water • Widely used preservative in the world • Effective against yeast, molds, and bacteria • Effective up to pH 6.5 • Maximum level allowable by law is ...
Asian Journal of Research in Chemistry
... concentration and was successfully used in monitoring left over drug. The detection limit of Rabeprazole sodium at a signal-tonoise ratio of 3 was 1.80ng/ml in human plasma while quantification limit in human serum was 5.60 ng/ml. The proposed method is applicable to routine analysis of Rabeprazole ...
... concentration and was successfully used in monitoring left over drug. The detection limit of Rabeprazole sodium at a signal-tonoise ratio of 3 was 1.80ng/ml in human plasma while quantification limit in human serum was 5.60 ng/ml. The proposed method is applicable to routine analysis of Rabeprazole ...
Tyrosinase Related Protein 75 (TRP75 / gp75) Ab-1
... this material have not been thoroughly investigated. Appropriate measures should be taken to avoid skin and eye contact, inhalation, and ingestion. The material contains 0.09% sodium azide as a preservative. Although the quantity of azide is very small, appropriate care should be taken when handling ...
... this material have not been thoroughly investigated. Appropriate measures should be taken to avoid skin and eye contact, inhalation, and ingestion. The material contains 0.09% sodium azide as a preservative. Although the quantity of azide is very small, appropriate care should be taken when handling ...