(CSIF) Clinical Site Information Form
... primary clinical site. Please note that if the satellite site(s) offering a clinical experience differs from the primary clinical site, a separate CSIF must be completed for each satellite site. Additionally, if any of the satellite sites have a different CCCE, an abbreviated resume must be complete ...
... primary clinical site. Please note that if the satellite site(s) offering a clinical experience differs from the primary clinical site, a separate CSIF must be completed for each satellite site. Additionally, if any of the satellite sites have a different CCCE, an abbreviated resume must be complete ...
Human Research Seminar Series How to Maintain Research Data Institutional
... • A protocol deviation/violation is generally an unplanned excursion from the protocol that is not implemented or intended as a systematic change. • Protocol deviation is also used to refer to any other, unplanned, instance(s) of protocol noncompliance. • For example: > Situations in which the inves ...
... • A protocol deviation/violation is generally an unplanned excursion from the protocol that is not implemented or intended as a systematic change. • Protocol deviation is also used to refer to any other, unplanned, instance(s) of protocol noncompliance. • For example: > Situations in which the inves ...
Treatment of Fibromyalgia Syndrome (FMS)
... Treatment of Fibromyalgia Syndrome (FMS) Fibromyalgia Syndrome (FMS) is characterized by chronic, widespread, non-inflammatory pain, fatigue, stiffness, sleep disturbances, and mood changes.1-3 Women are more commonly affected than men and an exact cause is largely unknown.3 Onset of fibromyalgia ha ...
... Treatment of Fibromyalgia Syndrome (FMS) Fibromyalgia Syndrome (FMS) is characterized by chronic, widespread, non-inflammatory pain, fatigue, stiffness, sleep disturbances, and mood changes.1-3 Women are more commonly affected than men and an exact cause is largely unknown.3 Onset of fibromyalgia ha ...
Attachment: Product Information Brivaracetam
... indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behaviour, and/or any unusual changes in mood or behaviour. Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomised ...
... indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behaviour, and/or any unusual changes in mood or behaviour. Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomised ...
the RAMPART trial - Department of Emergency Medicine
... also asked to state whether the subject is seizing on arrival at the ED. Post-treatment monitoring and treatment in the field. Patients are continuously assessed throughout the period of out-of-hospital management and transport, with vital signs measured at intervals of 5 minutes or less. Assessment ...
... also asked to state whether the subject is seizing on arrival at the ED. Post-treatment monitoring and treatment in the field. Patients are continuously assessed throughout the period of out-of-hospital management and transport, with vital signs measured at intervals of 5 minutes or less. Assessment ...
PDF - Bentham Open
... the R115777 drug at Fox Chase Cancer Center in Philadelphia, PA in 1999. We will use this study as an example to demonstrate the application of web-based online dose finding calculator. R115777 is a selective nonpeptidomimetic inhibitor of farnesyltransferase (FTase), one of several enzymes responsi ...
... the R115777 drug at Fox Chase Cancer Center in Philadelphia, PA in 1999. We will use this study as an example to demonstrate the application of web-based online dose finding calculator. R115777 is a selective nonpeptidomimetic inhibitor of farnesyltransferase (FTase), one of several enzymes responsi ...
Abstract #5067 The Nonclinical Toxicology Profile
... (MF). Pacri3nib is a potent inhibitor of wild-type and mutant isoforms of JAK2 and FLT3 (IC50 values < 15 nM), and it does not suppress JAK1 at clinically relevant concentra3ons. Addi3onal kinases targeted by pacri3nib and evaluated in a comprehensive kinome profile by Reac3on Biology Corp. inclu ...
... (MF). Pacri3nib is a potent inhibitor of wild-type and mutant isoforms of JAK2 and FLT3 (IC50 values < 15 nM), and it does not suppress JAK1 at clinically relevant concentra3ons. Addi3onal kinases targeted by pacri3nib and evaluated in a comprehensive kinome profile by Reac3on Biology Corp. inclu ...
... assignments needed to assess student growth. Faculty may require additional assignments and clinical work to ensure students have met clinical objectives. Students are expected to comply with any additional assignments or clinical hours assigned. Students are expected to prepare for clinical practic ...
Recommendations for whole genome sequencing * putting the cart
... Divergence on issue of what options should be offered 96% would return clinically actionable findings 52% of clinical genetics professionals would disclose adult onset conditions that are not clinically actionable (Lemke et al ...
... Divergence on issue of what options should be offered 96% would return clinically actionable findings 52% of clinical genetics professionals would disclose adult onset conditions that are not clinically actionable (Lemke et al ...
July 2015 - Subsequent decisions Not to Recommend (Word
... GSK believes that the application of the PBAC’s current HTA evaluation criteria will prohibit the adoption of a vaccine that prevents a rare and unpredictable life threatening disease, with devastating impact in children, adolescents and their families. To this end, GSK supports the PBAC Guidelines ...
... GSK believes that the application of the PBAC’s current HTA evaluation criteria will prohibit the adoption of a vaccine that prevents a rare and unpredictable life threatening disease, with devastating impact in children, adolescents and their families. To this end, GSK supports the PBAC Guidelines ...
Acetylcysteine
... Acetylcysteine (an antioxidant) represents a safe, non-expensive , easy to administer, and widely available drug THE EVIDENCE Low quality (few trials with allocation concealment, blinding, and ITT analysis) Low statistical power (median trial size = 80 patients) Uncertain effects on clinical endpoin ...
... Acetylcysteine (an antioxidant) represents a safe, non-expensive , easy to administer, and widely available drug THE EVIDENCE Low quality (few trials with allocation concealment, blinding, and ITT analysis) Low statistical power (median trial size = 80 patients) Uncertain effects on clinical endpoin ...
Treating Overactive Bladder in the Elderly: Side
... dizziness, headache, etc. But the clinical trials were structured to capture adverse effects reported by the patient and not to test for specific CNS adverse effects such as cognitive changes. In addition, these studies included a relatively small number of elderly patients with limited cognitive or ...
... dizziness, headache, etc. But the clinical trials were structured to capture adverse effects reported by the patient and not to test for specific CNS adverse effects such as cognitive changes. In addition, these studies included a relatively small number of elderly patients with limited cognitive or ...
Clinical Medicine Insights: Oncology emerging pharmacotherapy for
... second line treatment of those with either refractory or relapsed disease involves high dose therapy and autologous stem cell transplant (ASCT) following salvage chemotherapy. Whilst ASCT may be curative, it will be ineffective in up to 50% of patients.14 For older or less fit patients ASCT is not a ...
... second line treatment of those with either refractory or relapsed disease involves high dose therapy and autologous stem cell transplant (ASCT) following salvage chemotherapy. Whilst ASCT may be curative, it will be ineffective in up to 50% of patients.14 For older or less fit patients ASCT is not a ...
Policy XI.B
... 1. Food and Drug Administration (FDA): The FDA is the federal oversight agency responsible for protecting the public health by assuring the safety, efficacy, and security of human and veterinary drugs, biological products, medical devices, our nation’s food supply, cosmetics, and products that emit ...
... 1. Food and Drug Administration (FDA): The FDA is the federal oversight agency responsible for protecting the public health by assuring the safety, efficacy, and security of human and veterinary drugs, biological products, medical devices, our nation’s food supply, cosmetics, and products that emit ...
IND Application Template
... Note: Delete this sub-section if not applicable. Overview any FDA-approved marketed indications for the study drug. Reference to the FDA drug labeling for approved indications should be noted here, with copies of such labeling included in the attachment section of this IND application. Note: If the ...
... Note: Delete this sub-section if not applicable. Overview any FDA-approved marketed indications for the study drug. Reference to the FDA drug labeling for approved indications should be noted here, with copies of such labeling included in the attachment section of this IND application. Note: If the ...
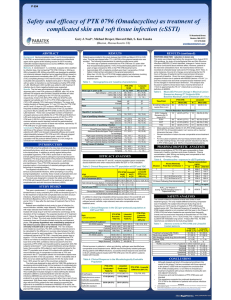
Safety and efficacy of PTK 0796 (Omadacycline) as treatment of
... Conclusions Results of this phase 3 trial experience are consistent with those of the phase 2 clinical program that also involved patients with cSSTI and showed comparable efficacy and overall safety/tolerability between PTK 0796 and LZD. Although stopped before meeting planned enrolment goals, resu ...
... Conclusions Results of this phase 3 trial experience are consistent with those of the phase 2 clinical program that also involved patients with cSSTI and showed comparable efficacy and overall safety/tolerability between PTK 0796 and LZD. Although stopped before meeting planned enrolment goals, resu ...
Supplementary File - Annals of the Rheumatic Diseases
... the end of follow-up (after 3 yrs.), the outcome was ACR remission for at least two months. Additionally, the authors studied the fulfilling of the Magnusson criteria11 (no steroids plus four of the following six criteria: morning stiffness < 30min, no fatigue, no joint pain, no joint tenderness or ...
... the end of follow-up (after 3 yrs.), the outcome was ACR remission for at least two months. Additionally, the authors studied the fulfilling of the Magnusson criteria11 (no steroids plus four of the following six criteria: morning stiffness < 30min, no fatigue, no joint pain, no joint tenderness or ...
Initial Management of Gastroenteritis
... Add 100ml/kg for children who were initially shocked, or 50 ml/kg for children who were not initially shocked, to maintenance fluid requirements Monitor clinical response Measure plasma sodium, potassium, urea, creatinine and glucose at the start, monitor regularly, and change fluid composition or r ...
... Add 100ml/kg for children who were initially shocked, or 50 ml/kg for children who were not initially shocked, to maintenance fluid requirements Monitor clinical response Measure plasma sodium, potassium, urea, creatinine and glucose at the start, monitor regularly, and change fluid composition or r ...
Access to unapproved biologicals
... The schemes apply to the testing of any biologicals not entered on the ARTG, including any new preparations or uses, as well as use of an included biological in a clinical trial beyond the conditions of its marketing approval. The clinical trial arrangements (for both schemes) are based on the same ...
... The schemes apply to the testing of any biologicals not entered on the ARTG, including any new preparations or uses, as well as use of an included biological in a clinical trial beyond the conditions of its marketing approval. The clinical trial arrangements (for both schemes) are based on the same ...
RSI for Reporting Period
... Refer to section 3.3 of ICH E2F This section should include a description of significant actions related to safety that have been taken during the reporting period. For example: Refusal to authorise a clinical trial for ethical or safety reasons; Partial or complete clinical trial suspension or earl ...
... Refer to section 3.3 of ICH E2F This section should include a description of significant actions related to safety that have been taken during the reporting period. For example: Refusal to authorise a clinical trial for ethical or safety reasons; Partial or complete clinical trial suspension or earl ...
Surrogate Endpoints: A Regulatory View
... – Predictive model for effectiveness may not address long-term safety (this is true for DES example as well) since “there is no surrogate for safety” (Temple, ...
... – Predictive model for effectiveness may not address long-term safety (this is true for DES example as well) since “there is no surrogate for safety” (Temple, ...
Kids denied monitors in AIDS drug trials
... the past two decades, often without providing them a basic protection afforded in federal law and required by some states, an Associated Press review has found. The research funded by the National Institutes of Health spanned the country. It was most widespread in the 1990s as foster care agencies s ...
... the past two decades, often without providing them a basic protection afforded in federal law and required by some states, an Associated Press review has found. The research funded by the National Institutes of Health spanned the country. It was most widespread in the 1990s as foster care agencies s ...
protocol synopsis
... This section should contain details of age, sex, disease, prior treatment constraints etc., under which a subject is deemed to be suitable (eligible) to participate in the trial. This also includes healthy volunteers and any “control” groups etc. Each such “group” should be defined separately. A sim ...
... This section should contain details of age, sex, disease, prior treatment constraints etc., under which a subject is deemed to be suitable (eligible) to participate in the trial. This also includes healthy volunteers and any “control” groups etc. Each such “group” should be defined separately. A sim ...
Safety Pharmacology for Oncology Pharmaceuticals at CDER
... cardiovascular, respiratory and central nervous systems, should be available before the initiation of clinical studies; such parameters could be included in general toxicology studies. Detailed clinical observations following dosing and appropriate electrocardiographic measurements in nonrodents are ...
... cardiovascular, respiratory and central nervous systems, should be available before the initiation of clinical studies; such parameters could be included in general toxicology studies. Detailed clinical observations following dosing and appropriate electrocardiographic measurements in nonrodents are ...
Understanding Clinical Trial Design
... more familiar they become with these areas, and the more comfortable they become with the language and style of scientific discourse, the more effective they will be in influencing the course of research. This tutorial follows from these assumptions. Generic questions that advocates can ask about vi ...
... more familiar they become with these areas, and the more comfortable they become with the language and style of scientific discourse, the more effective they will be in influencing the course of research. This tutorial follows from these assumptions. Generic questions that advocates can ask about vi ...