Tartrazine: a potentially hazardous dye in Canadian drugs.
... soaps, may all contain tartrazine. Pharmaceutical manufacturers use this dye in many types of drug products. Tablets and capsules may contain tartrazine in coatings, shells and excipient materials.8 Other dosage forms in which tartrazine may be found include liquids for ingestion, lozenges, ointment ...
... soaps, may all contain tartrazine. Pharmaceutical manufacturers use this dye in many types of drug products. Tablets and capsules may contain tartrazine in coatings, shells and excipient materials.8 Other dosage forms in which tartrazine may be found include liquids for ingestion, lozenges, ointment ...
Pharmacokinetic and pharmacodynamic drug interactions
... in vivo studies investigating such interactions were identified by searches using databases such as MEDLINE, EMBASE, BIOSIS, Australasian medical index (AMI) and international pharmaceutical abstracts. Search terms were kava, kawa, Piper methysticum, kavalactones, kava–drug and kava–herb interaction ...
... in vivo studies investigating such interactions were identified by searches using databases such as MEDLINE, EMBASE, BIOSIS, Australasian medical index (AMI) and international pharmaceutical abstracts. Search terms were kava, kawa, Piper methysticum, kavalactones, kava–drug and kava–herb interaction ...
Synthesis and in vitro activities of a new antiviral duplex drug linking
... for new effective antiviral agents is ongoing.1,2 The successful clinical application of new antiviral drugs, however, is often limited as compounds with high in vitro activity do not meet the high expectations made for drug development and licensing. An alternative to the search for new single agen ...
... for new effective antiviral agents is ongoing.1,2 The successful clinical application of new antiviral drugs, however, is often limited as compounds with high in vitro activity do not meet the high expectations made for drug development and licensing. An alternative to the search for new single agen ...
Safety Assessment Of Alumina And Aluminum Hydroxide As Used
... The Panel also concluded that the aluminum hydroxide used in cosmetics is chemically equivalent to that used in OTC antacid products. The FDA found that the information submitted for the approval of those drugs was adequate to support safe use. The FDA also determined that aluminum hydroxide is gene ...
... The Panel also concluded that the aluminum hydroxide used in cosmetics is chemically equivalent to that used in OTC antacid products. The FDA found that the information submitted for the approval of those drugs was adequate to support safe use. The FDA also determined that aluminum hydroxide is gene ...
Ropinirole 0.25 mg, 0.5 mg, 1 mg, 2 mg, 3 mg, 4 mg and 5 mg
... Synthesis of the active substance from the designated starting materials has been adequately described and appropriate in-process controls and intermediate specifications are applied. Satisfactory specifications are in place for all starting materials and reagents and these are supported by relevant ...
... Synthesis of the active substance from the designated starting materials has been adequately described and appropriate in-process controls and intermediate specifications are applied. Satisfactory specifications are in place for all starting materials and reagents and these are supported by relevant ...
Allergan to Acquire Naurex - McCormick School of Engineering
... decade, according to NeuroPerspective, a newsletter. This year so far, $4.3 billion has already been put into such firms, not including this deal. Among the beneficiaries have been neuroscience-based companies like Sage Therapeutics and Intracellular therapies. Discussions for the deal began late la ...
... decade, according to NeuroPerspective, a newsletter. This year so far, $4.3 billion has already been put into such firms, not including this deal. Among the beneficiaries have been neuroscience-based companies like Sage Therapeutics and Intracellular therapies. Discussions for the deal began late la ...
SCIENTIFIC DISCUSSION
... recognise many epileptic diseases or syndromes and each of them can be expressed clinically by one or several seizure groupings. Partial epilepsies (localisation related) are the more frequent, accounting for more than 60% of the epilepsies, and they include most of the difficult-to-treat patients. ...
... recognise many epileptic diseases or syndromes and each of them can be expressed clinically by one or several seizure groupings. Partial epilepsies (localisation related) are the more frequent, accounting for more than 60% of the epilepsies, and they include most of the difficult-to-treat patients. ...
Assessment report on hamamelis virginiana l - EMA
... America, found in damp woods ranging from Nova Scotia to Florida and as far west as Texas. It is cultivated on a small scale in Europe, though the material of commerce is obtained mainly from the eastern USA and Canada (Wichtl and Bisset, 1994). The flowers, bark and leaves of the common, colourful ...
... America, found in damp woods ranging from Nova Scotia to Florida and as far west as Texas. It is cultivated on a small scale in Europe, though the material of commerce is obtained mainly from the eastern USA and Canada (Wichtl and Bisset, 1994). The flowers, bark and leaves of the common, colourful ...
2009 St Johns Wort Quality Issues and Active Compounds
... Nielsen et al. and Baureithel et al. focused their investigations on the bifl avone amento-fl avone, which bound to the brain benzodiazepine receptors with an affi nity comparable to diazepam (18, 19). However, the fl avonoids rutin, hyperoside, and quercitrin did not inhibit benzodiazepine binding ...
... Nielsen et al. and Baureithel et al. focused their investigations on the bifl avone amento-fl avone, which bound to the brain benzodiazepine receptors with an affi nity comparable to diazepam (18, 19). However, the fl avonoids rutin, hyperoside, and quercitrin did not inhibit benzodiazepine binding ...
Final Amended Safety Assessment of Sodium Sulfate as Used in
... VCRP data obtained from the FDA in 2016 indicate that Sodium Sulfate is used in 777 cosmetic formulations6 compared to 28 uses in the 2000 assessment1 (Table 1). Frequencies of use notably increased since 2000 as follows (uses reported in 20166 vs. uses reported in 20001): 86 vs. 13 leave-on; 661 vs ...
... VCRP data obtained from the FDA in 2016 indicate that Sodium Sulfate is used in 777 cosmetic formulations6 compared to 28 uses in the 2000 assessment1 (Table 1). Frequencies of use notably increased since 2000 as follows (uses reported in 20166 vs. uses reported in 20001): 86 vs. 13 leave-on; 661 vs ...
5 1 Prereview - World Health Organization
... Lisdexamfetamine is a prodrug and an inactive molecule until ingestion. After oral administration, enzyme hydrolysis following contact with red blood cells will break lisdexamfetamine into L-lysine, a naturally occurring essential amino acid and active damphetamine which is responsible for the drug’ ...
... Lisdexamfetamine is a prodrug and an inactive molecule until ingestion. After oral administration, enzyme hydrolysis following contact with red blood cells will break lisdexamfetamine into L-lysine, a naturally occurring essential amino acid and active damphetamine which is responsible for the drug’ ...
Evaluation of the use of static and dynamic models to predict drug
... extraction and neglecting gut extraction in the case of induction interactions, performed better than Simcyp (84% compared to 58% of the interactions predicted within 2-fold). Differences in the prediction outcomes between the static and dynamic models are attributable to differences in first-pass c ...
... extraction and neglecting gut extraction in the case of induction interactions, performed better than Simcyp (84% compared to 58% of the interactions predicted within 2-fold). Differences in the prediction outcomes between the static and dynamic models are attributable to differences in first-pass c ...
PRODUCT MONOGRAPH PrMONUROL® Fosfomycin powder, 3g
... MONUROL to fasted patients, the total body clearance (CLBT) and mean renal clearance (CLR) of fosfomycin are 16.9 L/hr and 6.3 L/hr respectively. Approximately 38% of a MONUROL dose (equivalent to 3 g of fosfomycin) is recovered from urine and 18% is recovered from feces. A mean maximum urine fosfom ...
... MONUROL to fasted patients, the total body clearance (CLBT) and mean renal clearance (CLR) of fosfomycin are 16.9 L/hr and 6.3 L/hr respectively. Approximately 38% of a MONUROL dose (equivalent to 3 g of fosfomycin) is recovered from urine and 18% is recovered from feces. A mean maximum urine fosfom ...
PropoFlo - zoetisUS.com
... source of exposure and seek medical attention. Respiratory depression should be treated by artificial ventilation and oxygen. ...
... source of exposure and seek medical attention. Respiratory depression should be treated by artificial ventilation and oxygen. ...
... glass throat were used to determine these distributions. The QCM provided nearly real-time distributions for the mass and radiolabel on the day of the study. The Andersen samplers provided drug and radiolabel distributions at a later date for verification. Before the labelling procedure began, the c ...
Safety Assessment Of Alumina And Aluminum Hydroxide As Used
... Definitions, CAS Nos., and functions are provided in Table 1. The structures of alumina and aluminum hydroxide are provided in Figure 1. Alumina, also known as aluminum oxide (Al 2 O 3 ), is dehydrated (or calcined) aluminum hydroxide.1 Alumina is also the primary constituent of emerald, ruby, and s ...
... Definitions, CAS Nos., and functions are provided in Table 1. The structures of alumina and aluminum hydroxide are provided in Figure 1. Alumina, also known as aluminum oxide (Al 2 O 3 ), is dehydrated (or calcined) aluminum hydroxide.1 Alumina is also the primary constituent of emerald, ruby, and s ...
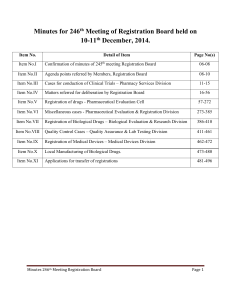
Minutes of 246th meeting of Registration Board
... Director QA < opined that for export purpose only those formulations should be registered, which are already registered in Pakistan. For new fornulations (which are not registered in Pakistan), manufacturers should first conduct stability studies and then registration for export purpose be granted ...
... Director QA < opined that for export purpose only those formulations should be registered, which are already registered in Pakistan. For new fornulations (which are not registered in Pakistan), manufacturers should first conduct stability studies and then registration for export purpose be granted ...
Codeine use in children and ultra-rapid metabolisers
... products in Australia. This is in contrast to other major jurisdictions including the United States, European Union and Canada. Codeine is a commonly used medication that may be perceived by the Australian public to be very safe, especially in light of its availability in OTC preparations. Therefore ...
... products in Australia. This is in contrast to other major jurisdictions including the United States, European Union and Canada. Codeine is a commonly used medication that may be perceived by the Australian public to be very safe, especially in light of its availability in OTC preparations. Therefore ...