Lecture05: Electric Potential
... 5-1: In the four examples shown in the sketch, a force F an object and does work. In all four cases, the force has same magnitude and the displacement of the object is to right and has the same magnitude. Rank the cases in order of the work done by the force on object, from most positive to the most ...
... 5-1: In the four examples shown in the sketch, a force F an object and does work. In all four cases, the force has same magnitude and the displacement of the object is to right and has the same magnitude. Rank the cases in order of the work done by the force on object, from most positive to the most ...
electric circuit
... The Ss are divided into groups for three or four students and make an electric circuit comprising a battery, an electric resistance and a bulb (Electric circuit activity). The Ss analyze and summarize the results of your experiment, they list any questions you still have about your experiment and th ...
... The Ss are divided into groups for three or four students and make an electric circuit comprising a battery, an electric resistance and a bulb (Electric circuit activity). The Ss analyze and summarize the results of your experiment, they list any questions you still have about your experiment and th ...
101603.kung.strain_v..
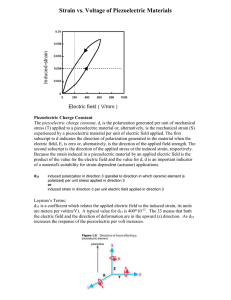
... stress (T) applied to a piezoelectric material or, alternatively, is the mechanical strain (S) experienced by a piezoelectric material per unit of electric field applied. The first subscript to d indicates the direction of polarization generated in the material when the electric field, E, is zero or ...
... stress (T) applied to a piezoelectric material or, alternatively, is the mechanical strain (S) experienced by a piezoelectric material per unit of electric field applied. The first subscript to d indicates the direction of polarization generated in the material when the electric field, E, is zero or ...
It is sometimes difficult to find the polarity of an
... 12 volts, a charged capacitor has the same potential difference across the plates as the source. ...
... 12 volts, a charged capacitor has the same potential difference across the plates as the source. ...
Document
... One never directly measures dose with an ion chamber. -- one measures ionizations only (neglecting excitations, but W / e has this implicitly included, yet we see the complications with this too (i.e., humidity). -- even when correcting for recombination, we are still not even directly measuring cha ...
... One never directly measures dose with an ion chamber. -- one measures ionizations only (neglecting excitations, but W / e has this implicitly included, yet we see the complications with this too (i.e., humidity). -- even when correcting for recombination, we are still not even directly measuring cha ...
File
... The nucleus is about 99.9% of the mass of the atom This is because a proton or neutron has about 2000X more mass than an electron! The number of protons contained in the nucleus determines what kind of atom it is ...
... The nucleus is about 99.9% of the mass of the atom This is because a proton or neutron has about 2000X more mass than an electron! The number of protons contained in the nucleus determines what kind of atom it is ...
Atoms and Ions
... allowed to fall between two charged plates where they were then observed, visually. The air inside the chamber was exposed to x-rays, which displace electrons from air molecules resulting in both negatively charged electrons and positively charged air molecules. These species come in contact with th ...
... allowed to fall between two charged plates where they were then observed, visually. The air inside the chamber was exposed to x-rays, which displace electrons from air molecules resulting in both negatively charged electrons and positively charged air molecules. These species come in contact with th ...
Sir Joseph John “J
... particles of matter, but Thomson proved them wrong when he discovered that atoms contained particles known as electrons. Thomson discovered this through his explorations on the properties of cathode rays. Thomson found that the rays could be deflected by an electric field (in addition to magnetic fi ...
... particles of matter, but Thomson proved them wrong when he discovered that atoms contained particles known as electrons. Thomson discovered this through his explorations on the properties of cathode rays. Thomson found that the rays could be deflected by an electric field (in addition to magnetic fi ...
Document
... Last lecture: 1. Properties of the electric field, field lines 2. Conductors in electrostatic equilibrium Electric field is zero everywhere within the conductor. ...
... Last lecture: 1. Properties of the electric field, field lines 2. Conductors in electrostatic equilibrium Electric field is zero everywhere within the conductor. ...
Phy102 L_EquiPotential
... (equal) at all points along its path. Try several other values of equipotentials between 0.0 and 6 Volts, evenly separated in potential, carrying each line as far as you can. Make sure that you have enough values to draw several equipotential lines each an equal V from the other. ...
... (equal) at all points along its path. Try several other values of equipotentials between 0.0 and 6 Volts, evenly separated in potential, carrying each line as far as you can. Make sure that you have enough values to draw several equipotential lines each an equal V from the other. ...
Electric charge
Electric charge is the physical property of matter that causes it to experience a force when placed in an electromagnetic field. There are two types of electric charges: positive and negative. Positively charged substances are repelled from other positively charged substances, but attracted to negatively charged substances; negatively charged substances are repelled from negative and attracted to positive. An object is negatively charged if it has an excess of electrons, and is otherwise positively charged or uncharged. The SI derived unit of electric charge is the coulomb (C), although in electrical engineering it is also common to use the ampere-hour (Ah), and in chemistry it is common to use the elementary charge (e) as a unit. The symbol Q is often used to denote charge. The early knowledge of how charged substances interact is now called classical electrodynamics, and is still very accurate if quantum effects do not need to be considered.The electric charge is a fundamental conserved property of some subatomic particles, which determines their electromagnetic interaction. Electrically charged matter is influenced by, and produces, electromagnetic fields. The interaction between a moving charge and an electromagnetic field is the source of the electromagnetic force, which is one of the four fundamental forces (See also: magnetic field).Twentieth-century experiments demonstrated that electric charge is quantized; that is, it comes in integer multiples of individual small units called the elementary charge, e, approximately equal to 6981160200000000000♠1.602×10−19 coulombs (except for particles called quarks, which have charges that are integer multiples of e/3). The proton has a charge of +e, and the electron has a charge of −e. The study of charged particles, and how their interactions are mediated by photons, is called quantum electrodynamics.