Scanning Electron Microscopy / Electron Probe X
... incident electron that transfers energy to an electron of the sample. This excited electron then leaves the sample with a very small kinetic energy. Due to this low energy, only SE’s that are created near the surface can exit the sample and can be detected. Any variation in topography of the surface ...
... incident electron that transfers energy to an electron of the sample. This excited electron then leaves the sample with a very small kinetic energy. Due to this low energy, only SE’s that are created near the surface can exit the sample and can be detected. Any variation in topography of the surface ...
Electrons in atoms practice test File
... ____ 15. Emission of light from an atom occurs when an electron ____. a. drops from a higher to a lower energy level b. jumps from a lower to a higher energy level c. moves within its atomic orbital d. falls into the nucleus ____ 16. The quantum mechanical model of the atom ____. a. defines the exac ...
... ____ 15. Emission of light from an atom occurs when an electron ____. a. drops from a higher to a lower energy level b. jumps from a lower to a higher energy level c. moves within its atomic orbital d. falls into the nucleus ____ 16. The quantum mechanical model of the atom ____. a. defines the exac ...
O usually has oxidation number of -2, except in peroxides where it is
... The sum of the oxidation numbers of the elements in a polyatomic ion must equal the ion charge. Consider these examples. If there are two poly atomic ions in a compound deal with them first. ...
... The sum of the oxidation numbers of the elements in a polyatomic ion must equal the ion charge. Consider these examples. If there are two poly atomic ions in a compound deal with them first. ...
Build an Atom
... Procedure: Play with the Sims Chemistry Build An Atom Begin by playing with the simulation for a while. Become familiar with the interface. What happens when you add protons, neutrons, or electrons? To start over, click Show the symbol, atomic mass, and charge by clicking on the ...
... Procedure: Play with the Sims Chemistry Build An Atom Begin by playing with the simulation for a while. Become familiar with the interface. What happens when you add protons, neutrons, or electrons? To start over, click Show the symbol, atomic mass, and charge by clicking on the ...
Chapter 1 - Manual Science Chemistry/Physics
... The periodic table organizes elements by their chemical properties o Elements serve as the building blocks of matter. o Elements cannot be decomposed by chemical changes o Each element has characteristic properties o The vertical columns of the periodic table are called groups, or families. Each ...
... The periodic table organizes elements by their chemical properties o Elements serve as the building blocks of matter. o Elements cannot be decomposed by chemical changes o Each element has characteristic properties o The vertical columns of the periodic table are called groups, or families. Each ...
Chemistry I - Net Start Class
... d. Atoms unite in definite ratios to form compounds. 74. Which of these statements is NOT true? a. atoms of the same element can have different masses b. atoms of isotopes of an element have different numbers of protons c. the nucleus of an atom has a positive charge d. atoms are mostly empty space ...
... d. Atoms unite in definite ratios to form compounds. 74. Which of these statements is NOT true? a. atoms of the same element can have different masses b. atoms of isotopes of an element have different numbers of protons c. the nucleus of an atom has a positive charge d. atoms are mostly empty space ...
Atomic Structure
... number of protons in the nucleus Also the number of electrons in a neutral atom Located just above the element symbol on the PT (whole number) Elements are listed on the PT according to their atomic number increasing from left to right ...
... number of protons in the nucleus Also the number of electrons in a neutral atom Located just above the element symbol on the PT (whole number) Elements are listed on the PT according to their atomic number increasing from left to right ...
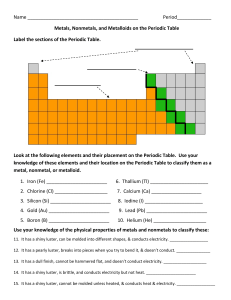
Name Period_____________ Metals, Nonmetals, and Metalloids on
... Metals, Nonmetals, and Metalloids on the Periodic Table Label the sections of the Periodic Table. ...
... Metals, Nonmetals, and Metalloids on the Periodic Table Label the sections of the Periodic Table. ...
Biochemistry-Review of the Basics
... Leads to high boiling point- keeps earth cool b/c bodies of water absorb a lot of heat without significant temperature change ...
... Leads to high boiling point- keeps earth cool b/c bodies of water absorb a lot of heat without significant temperature change ...
Final Exam Review Guide
... Describe the “Kinetic Theory of Gases” and list the three assumptions associated with it. What volume does one mole of any gas occupy at STP? 22.4 L Kinetic theory states that all matter is composed of particles and the particles are in constant motion. Particles are small hard spheres which are not ...
... Describe the “Kinetic Theory of Gases” and list the three assumptions associated with it. What volume does one mole of any gas occupy at STP? 22.4 L Kinetic theory states that all matter is composed of particles and the particles are in constant motion. Particles are small hard spheres which are not ...
Atomic Theory
... Bohr suggested that electrons surround the nucleus in specific energy levels called energy shells ...
... Bohr suggested that electrons surround the nucleus in specific energy levels called energy shells ...
Chapter 4 test review
... ____ 14. What is the difference between an atom in the ground state and an atom in an excited state? a. The atom in the ground state has less energy and is less stable than the atom in an excited state. b. The atom in an excited state has one fewer electron than the atom in the ground state. c. The ...
... ____ 14. What is the difference between an atom in the ground state and an atom in an excited state? a. The atom in the ground state has less energy and is less stable than the atom in an excited state. b. The atom in an excited state has one fewer electron than the atom in the ground state. c. The ...
Midterm Review Answers
... Questions 52-53nrefer to the following types of energy A) Activation energy B) Free energy C) Ionization energy D) Kinetic energy E) Lattice energy 52. The energy required to convert a ground-state atom in the gas phase to a gaseous positive ion. C 53. The energy released when gas phase ions bond t ...
... Questions 52-53nrefer to the following types of energy A) Activation energy B) Free energy C) Ionization energy D) Kinetic energy E) Lattice energy 52. The energy required to convert a ground-state atom in the gas phase to a gaseous positive ion. C 53. The energy released when gas phase ions bond t ...
Education TI - Texas Instruments
... nucleus comprised of protons and neutrons surrounded by electrons. In this model, electrons orbit the nucleus in circular paths at different distances called electron shells. This model became popular because it fit the experimental results for Hydrogen. Later, the application of the model to heavie ...
... nucleus comprised of protons and neutrons surrounded by electrons. In this model, electrons orbit the nucleus in circular paths at different distances called electron shells. This model became popular because it fit the experimental results for Hydrogen. Later, the application of the model to heavie ...
Summary of lesson
... nucleus comprised of protons and neutrons surrounded by electrons. In this model, electrons orbit the nucleus in circular paths at different distances called electron shells. This model became popular because it fit the experimental results for Hydrogen. Later, the application of the model to heavie ...
... nucleus comprised of protons and neutrons surrounded by electrons. In this model, electrons orbit the nucleus in circular paths at different distances called electron shells. This model became popular because it fit the experimental results for Hydrogen. Later, the application of the model to heavie ...
Mongar Higher Secondary School
... ( empirical formula, salt, ionization, cation, molecular formula, base, acid, anion, dissociation, mercury, oxygen, sodium) i) A base reacts with an acid to form a …………and water only. ii) Negatively charged ion is called………. iii) ………….is a chemical formula which gives the simple whole number of diff ...
... ( empirical formula, salt, ionization, cation, molecular formula, base, acid, anion, dissociation, mercury, oxygen, sodium) i) A base reacts with an acid to form a …………and water only. ii) Negatively charged ion is called………. iii) ………….is a chemical formula which gives the simple whole number of diff ...
Chapter 5
... elements in that group are alkali metals. The alkali metals all have one valence electron. That similarity is what makes them behave the same chemically. They are very reactive. Reactivity is highest on the outer edges of the table and elements get less reactive the closer they are to the center ...
... elements in that group are alkali metals. The alkali metals all have one valence electron. That similarity is what makes them behave the same chemically. They are very reactive. Reactivity is highest on the outer edges of the table and elements get less reactive the closer they are to the center ...
Electronic Structure of Atoms
... • Bohr Model does not work for multi-electron atoms • Could not account for the intensities or the fine structure of the spectral lines (for example, in magnetic fields). • Today’s atomic model is based on the principles of wave ...
... • Bohr Model does not work for multi-electron atoms • Could not account for the intensities or the fine structure of the spectral lines (for example, in magnetic fields). • Today’s atomic model is based on the principles of wave ...
Modern Theories of Matter
... p the shadow showed that the light came from the cathode end of the tube p Crookes called the source of the light cathode rays q J.J. Thomson showed that cathode rays were the paths of negatively charged particles he called electrons q The Thomson Model p Protons had much more mass than electrons p ...
... p the shadow showed that the light came from the cathode end of the tube p Crookes called the source of the light cathode rays q J.J. Thomson showed that cathode rays were the paths of negatively charged particles he called electrons q The Thomson Model p Protons had much more mass than electrons p ...
The Periodic Table
... Atoms of the same element always have the same number of protons. This identifies them as the element that they are. But all atoms of an element don’t have to have the same number of neutrons. For example, all boron atoms have 5 protons. However, four-fifths of them have 6 neutrons and one-fifth of ...
... Atoms of the same element always have the same number of protons. This identifies them as the element that they are. But all atoms of an element don’t have to have the same number of neutrons. For example, all boron atoms have 5 protons. However, four-fifths of them have 6 neutrons and one-fifth of ...
Station 2: Atomic Models
... bombarded gold foil with alpha particles (Helium nuclei). A source which undergoes alpha decay is placed in a lead box with a small hole in it. Any of the alpha particles which hit the inside of the box are simply stopped by the box. Only those which pass through the opening are allowed to escape, a ...
... bombarded gold foil with alpha particles (Helium nuclei). A source which undergoes alpha decay is placed in a lead box with a small hole in it. Any of the alpha particles which hit the inside of the box are simply stopped by the box. Only those which pass through the opening are allowed to escape, a ...
Lecture 4
... • Two or more substances in different proportions • Substances that can be separated by physical methods Example: Pasta and water can be separated with a strainer. ...
... • Two or more substances in different proportions • Substances that can be separated by physical methods Example: Pasta and water can be separated with a strainer. ...