Study Guide (Semester 2)
... 1. Explain why electronegativity decreases as you go down the periodic table. (Must include shielding effect, energy levels, and valence electrons in your response.) ...
... 1. Explain why electronegativity decreases as you go down the periodic table. (Must include shielding effect, energy levels, and valence electrons in your response.) ...
Atomic Models
... The electrons mixed with the positive material, giving the atom a neutral charge. ...
... The electrons mixed with the positive material, giving the atom a neutral charge. ...
June review January 2012 part A
... (l) A neutral nucleus is surrounded by one or more negatively charged electrons. (2) A neutral nucleus is surrounded by one or more positively charged electrons. (3) A positively charged nucleus is surrounded by one or more negatively charged electrons. (4) A positively charged nucleus is surrounded ...
... (l) A neutral nucleus is surrounded by one or more negatively charged electrons. (2) A neutral nucleus is surrounded by one or more positively charged electrons. (3) A positively charged nucleus is surrounded by one or more negatively charged electrons. (4) A positively charged nucleus is surrounded ...
O: You will be able to explain how atoms make up the world.
... that makes up most matter. They are so small a piece of human hair is 1,000,000 thick. ...
... that makes up most matter. They are so small a piece of human hair is 1,000,000 thick. ...
Stoichiometry and Balanced Reactions Chemical Accounting
... – Fortunately, stoichiometric ratios apply for larger numbers of molecules (dozens, hundreds, millions…and more – In the laboratory, it is more practical to do things in terms of mass or volume, which are easy to measure. • How might we translate from one to the other? • Mass of an atom (atomic mass ...
... – Fortunately, stoichiometric ratios apply for larger numbers of molecules (dozens, hundreds, millions…and more – In the laboratory, it is more practical to do things in terms of mass or volume, which are easy to measure. • How might we translate from one to the other? • Mass of an atom (atomic mass ...
110 REVIEW MATERIALTro 2011
... Diatomic Elementsare those elements that exists as two atoms bonded together Representative elementsare "A" group elements Metals are those elements which have the characteristic properities of: high luster, good conductors of heat and electricity, and are malleable Nonmetals are those elements, unl ...
... Diatomic Elementsare those elements that exists as two atoms bonded together Representative elementsare "A" group elements Metals are those elements which have the characteristic properities of: high luster, good conductors of heat and electricity, and are malleable Nonmetals are those elements, unl ...
The History of the Atom
... Almost always occurs with alpha or beta decay. Does not change mass number or atomic number. No new element is created ...
... Almost always occurs with alpha or beta decay. Does not change mass number or atomic number. No new element is created ...
Lab 1.2
... Understanding different Models of the Hydrogen Atom: 1. Now that you’ve theorized about what is happening to the photons of energy, hi-light the “Prediction” button and observe other scientist’s theories about the atom. When you are working on this section, make comparisons by Using a wavelength o ...
... Understanding different Models of the Hydrogen Atom: 1. Now that you’ve theorized about what is happening to the photons of energy, hi-light the “Prediction” button and observe other scientist’s theories about the atom. When you are working on this section, make comparisons by Using a wavelength o ...
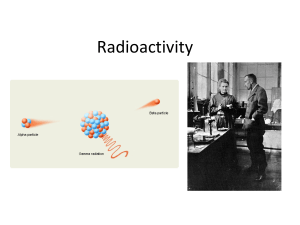
Radioactivity2015
... more elements. In any compound, the atoms of the elements are joined in a definite whole number ratio (1:1, 1:2, 3:2, etc) ...
... more elements. In any compound, the atoms of the elements are joined in a definite whole number ratio (1:1, 1:2, 3:2, etc) ...
Chapter 2. Atoms, Molecules, and Ions Common Student
... Radioactivity is the spontaneous emission of radiation. Consider the following experiment: • A radioactive substance is placed in a lead shield containing a small hole so that a beam of radiation is emitted from the shield. • The radiation is passed between two electrically charged plates and detect ...
... Radioactivity is the spontaneous emission of radiation. Consider the following experiment: • A radioactive substance is placed in a lead shield containing a small hole so that a beam of radiation is emitted from the shield. • The radiation is passed between two electrically charged plates and detect ...
FE Exam review for Chemistry
... Rutherford proved that protons & neutrons form a central nucleus, and that electrons surrounded the nucleus in a diffuse cloud. The Bohr or planetary model of the atom? Bohr believed that electrons circled the nucleus only at specific, or principle, energy levels. Like planets orbiting the nucleus, ...
... Rutherford proved that protons & neutrons form a central nucleus, and that electrons surrounded the nucleus in a diffuse cloud. The Bohr or planetary model of the atom? Bohr believed that electrons circled the nucleus only at specific, or principle, energy levels. Like planets orbiting the nucleus, ...
atomos
... the quantum model of the atom. In this model, the nucleus is orbited by a cloud of electrons, which are in different energy levels. ...
... the quantum model of the atom. In this model, the nucleus is orbited by a cloud of electrons, which are in different energy levels. ...
Chapter 4 powerpoint
... • Unstable nuclei lose energy by emitting radiation in a spontaneous process called radioactive decay. • Unstable radioactive elements undergo radioactive decay thus forming stable nonradioactive elements. ...
... • Unstable nuclei lose energy by emitting radiation in a spontaneous process called radioactive decay. • Unstable radioactive elements undergo radioactive decay thus forming stable nonradioactive elements. ...
Atomic Structure
... • The mass of an electron is so small that we often approximate it to zero. • The size and the sign are both important for the relative charges. • The absolute values for mass and charge are extremely small. We use the relative values because they are more convenient. ...
... • The mass of an electron is so small that we often approximate it to zero. • The size and the sign are both important for the relative charges. • The absolute values for mass and charge are extremely small. We use the relative values because they are more convenient. ...
Rxn Pred students
... On the AP exam: If the reaction is a combustion reaction and you don’t know the chemical formula for the hydrocarbon, make up a chemical formula and complete the reaction. You will earn partial points. ...
... On the AP exam: If the reaction is a combustion reaction and you don’t know the chemical formula for the hydrocarbon, make up a chemical formula and complete the reaction. You will earn partial points. ...
Chapters 6, 8
... If first atom is metal - ionic compound: 1. Find charges of both ions (from position in periodic table); write cation and anion with charges. 2. The sum of charges must be zero. Find out how many of each ion you must have. 3. Put index next to each ion indicating how many ions of that kind there is ...
... If first atom is metal - ionic compound: 1. Find charges of both ions (from position in periodic table); write cation and anion with charges. 2. The sum of charges must be zero. Find out how many of each ion you must have. 3. Put index next to each ion indicating how many ions of that kind there is ...
Chapter 3 - Industrial ISD
... 4. Which scientist discovered the electrons? 5. Which scientist discovered the nucleus? 6. Which scientist discovered that electrons where in energy levels? 7. Which scientist said atoms where neutral because they had equal number of protons and electrons? ...
... 4. Which scientist discovered the electrons? 5. Which scientist discovered the nucleus? 6. Which scientist discovered that electrons where in energy levels? 7. Which scientist said atoms where neutral because they had equal number of protons and electrons? ...
Chemical Equations Balancing Chemical Equations Try One…
... reaction type. In a chemical reaction, only 2 things are conserved the number of atoms and the conserved... number of grams. an arrow is used to separate reactants (the starting substances) and the products (what is made), the arrow is the same as an “equals sign” (=) in math for the number of e ...
... reaction type. In a chemical reaction, only 2 things are conserved the number of atoms and the conserved... number of grams. an arrow is used to separate reactants (the starting substances) and the products (what is made), the arrow is the same as an “equals sign” (=) in math for the number of e ...
Chapter 2 Powerpoint
... The method for naming alkynes is analogous to the naming of alkenes. However, the suffix is -yne rather than -ene. ...
... The method for naming alkynes is analogous to the naming of alkenes. However, the suffix is -yne rather than -ene. ...
end of year review
... _____ 7. Which of the following trends in the periodic table should be expected as the atomic number of the halogens increases from fluorine (F) to iodine (I)? a. Atomic radius decreases ...
... _____ 7. Which of the following trends in the periodic table should be expected as the atomic number of the halogens increases from fluorine (F) to iodine (I)? a. Atomic radius decreases ...
AP Chemistry Summer Assignment
... 15. Define the words: atomic number, atomic mass, mass number, molecular formula, structural formula, empirical formula, isotopes, cation, anion, metalloid, allotrope, stoichiometry. 16. White gold is an alloy that typically contains 60.0% by mass gold and the remainder is platinum. If 175 g of gold ...
... 15. Define the words: atomic number, atomic mass, mass number, molecular formula, structural formula, empirical formula, isotopes, cation, anion, metalloid, allotrope, stoichiometry. 16. White gold is an alloy that typically contains 60.0% by mass gold and the remainder is platinum. If 175 g of gold ...