112 ex iii lec outline f 04
... 4 Prefixes that give the number of ligands are not considered indetermining the alphabetical order 5 The names of anionic ligands end in the letter “o” 6 Neutral ligands generally have the molecule name Exception are water and ammonia 7 A greek prefix (di,tri,tetra, penta, and hexa) is used to indic ...
... 4 Prefixes that give the number of ligands are not considered indetermining the alphabetical order 5 The names of anionic ligands end in the letter “o” 6 Neutral ligands generally have the molecule name Exception are water and ammonia 7 A greek prefix (di,tri,tetra, penta, and hexa) is used to indic ...
Ch:3
... 3. If two or more degenerate orbitals are available, follow Hund’s rule. Hund’s Rule: If two or more orbitals with the same energy are available, one electron goes into each until all are half-full. The electrons in the half-filled orbitals all have the same value of their spin quantum number. Chapt ...
... 3. If two or more degenerate orbitals are available, follow Hund’s rule. Hund’s Rule: If two or more orbitals with the same energy are available, one electron goes into each until all are half-full. The electrons in the half-filled orbitals all have the same value of their spin quantum number. Chapt ...
BAT
... Important ideas: shielding, nuclear charge, definition of ionization energy, definition of electronegativity 10. Fill in the table below to identify the proper element Description Element symbol Alkali metal in period 3 Halogen in period 2 Any transition metal in period 5 Noble gas in period 4 Alkal ...
... Important ideas: shielding, nuclear charge, definition of ionization energy, definition of electronegativity 10. Fill in the table below to identify the proper element Description Element symbol Alkali metal in period 3 Halogen in period 2 Any transition metal in period 5 Noble gas in period 4 Alkal ...
Bacteria and Virus Research Jigsaw
... An equation is balanced when the number of atoms in the reactants are = to the number of atoms in the products ...
... An equation is balanced when the number of atoms in the reactants are = to the number of atoms in the products ...
1 ChE 505 WORKSHOP 1 1. Why are chemical reactions important
... What is the relationship between the initial moles of reactants and products, the moles for each of the above after some reaction time, the stoichiometric coefficients and reaction extent? ...
... What is the relationship between the initial moles of reactants and products, the moles for each of the above after some reaction time, the stoichiometric coefficients and reaction extent? ...
Document
... An equation is balanced when the number of atoms in the reactants are = to the number of atoms in the products ...
... An equation is balanced when the number of atoms in the reactants are = to the number of atoms in the products ...
Matter - HCC Learning Web
... • All substances have kinetic energy no matter what physical state they are in. • Solids have the lowest kinetic energy, and gases have the greatest kinetic energy. • As you increase the temperature of a substance, its kinetic energy increases. ...
... • All substances have kinetic energy no matter what physical state they are in. • Solids have the lowest kinetic energy, and gases have the greatest kinetic energy. • As you increase the temperature of a substance, its kinetic energy increases. ...
Preview Sample 1
... D) are always some form of carbohydrate. E) are naturally similar to sugars. 102) Alaska Natives have a lower incidence of heart disease even though their diets are high in fat and cholesterol. This may be due to the large amount of ________ in their diets. A) steroids B) omega-3 fatty acids C) trig ...
... D) are always some form of carbohydrate. E) are naturally similar to sugars. 102) Alaska Natives have a lower incidence of heart disease even though their diets are high in fat and cholesterol. This may be due to the large amount of ________ in their diets. A) steroids B) omega-3 fatty acids C) trig ...
Chapter 4 - Field Local Schools
... Beneath Famous to work with hands did not experiment Greeks settled disagreements by argument Aristotle was more famous He won His ideas carried through middle ages. Alchemists change lead to gold ...
... Beneath Famous to work with hands did not experiment Greeks settled disagreements by argument Aristotle was more famous He won His ideas carried through middle ages. Alchemists change lead to gold ...
Microanalysis in Electron Microscopy (EDS and WDS)
... dispersive spectrometers (EDS) sort the X-rays based on their energy; while wavelength dispersive spectrometers (WDS) sort the X-rays based on their wavelengths. WDS systems use X-ray diffraction as the means by which they separate X-rays of different wavelengths. The spectrometer consists of an ana ...
... dispersive spectrometers (EDS) sort the X-rays based on their energy; while wavelength dispersive spectrometers (WDS) sort the X-rays based on their wavelengths. WDS systems use X-ray diffraction as the means by which they separate X-rays of different wavelengths. The spectrometer consists of an ana ...
Lecture 8
... How many moles of hydrogen atoms are present in 39.0 g of C6H6? A. ½ mole of H atoms ...
... How many moles of hydrogen atoms are present in 39.0 g of C6H6? A. ½ mole of H atoms ...
Document
... Exceptions to the Aufbau Principle • Remember d and f orbitals require LARGE amounts of energy • If we can’t fill these sublevels, then the next best thing is to be HALF full (one electron in each orbital in the sublevel) • There are many exceptions, but the most common ones are d4 and d9 For the pu ...
... Exceptions to the Aufbau Principle • Remember d and f orbitals require LARGE amounts of energy • If we can’t fill these sublevels, then the next best thing is to be HALF full (one electron in each orbital in the sublevel) • There are many exceptions, but the most common ones are d4 and d9 For the pu ...
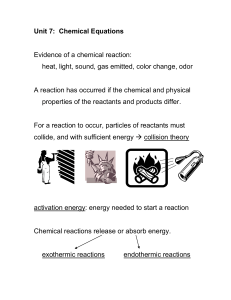
chemeqohnotes18f2005
... For a reaction to occur, particles of reactants must collide, and with sufficient energy collision theory ...
... For a reaction to occur, particles of reactants must collide, and with sufficient energy collision theory ...
Atom
... such a way that there are patterns of elements placed close together that have similar properties. For example, knowing the properties of one element in a column of the periodic table will help a person predict the properties of other elements in that same column. –Describe two properties common to ...
... such a way that there are patterns of elements placed close together that have similar properties. For example, knowing the properties of one element in a column of the periodic table will help a person predict the properties of other elements in that same column. –Describe two properties common to ...
Worksheet 8 Notes - Department of Chemistry | Oregon State
... What is a Lewis base? What is a Lewis acid? Let me start by stating that we are familiar with many bases and acids. Those we know to be bases are Lewis bases and those we know to be acids are Lewis acids. Our previous ideas of bases and acids came from Arrhenius, Bronsted, and Lowry. These ideas inv ...
... What is a Lewis base? What is a Lewis acid? Let me start by stating that we are familiar with many bases and acids. Those we know to be bases are Lewis bases and those we know to be acids are Lewis acids. Our previous ideas of bases and acids came from Arrhenius, Bronsted, and Lowry. These ideas inv ...
atomic model history
... Rutherford reasoned that if Thomson's model was correct then the mass of the atom was spread out throughout the atom. Then, if he shot high velocity alpha particles (helium nuclei) at an atom then there would be very little to deflect the alpha particles. He decided to test this with a thin film of ...
... Rutherford reasoned that if Thomson's model was correct then the mass of the atom was spread out throughout the atom. Then, if he shot high velocity alpha particles (helium nuclei) at an atom then there would be very little to deflect the alpha particles. He decided to test this with a thin film of ...
Atomic Structure
... In this question you will be assessed on using good English, organising information clearly and using specialist terms where appropriate. Describe a method for making pure crystals of magnesium chloride from magnesium and dilute hydrochloric acid. In your method you should name the apparatus you wil ...
... In this question you will be assessed on using good English, organising information clearly and using specialist terms where appropriate. Describe a method for making pure crystals of magnesium chloride from magnesium and dilute hydrochloric acid. In your method you should name the apparatus you wil ...
+ H 2 O(l)
... • Activity series can be used to predict reactions between metals and metal salts or acids. ...
... • Activity series can be used to predict reactions between metals and metal salts or acids. ...
Practice Test 1 (Chapters 1-7)
... 26. Which of the following processes is a chemical e. 6 change? 32. Which atomic particle determines the chemical a. Dry ice sublimes when left on the demo behavior of an atom? table in lecture. a. proton b. The light on a candle burns until a bell jar is b. electron placed over it for a period of t ...
... 26. Which of the following processes is a chemical e. 6 change? 32. Which atomic particle determines the chemical a. Dry ice sublimes when left on the demo behavior of an atom? table in lecture. a. proton b. The light on a candle burns until a bell jar is b. electron placed over it for a period of t ...
Chapter 3 – Atomic Structure and Properties
... The five 2p valence electrons of fluorine experience a highly positive effective nuclear charge of 5.2 and a Z2 very low n quantum number, so the value for eff is quite large. Thus, the energy of the valence orbitals n2 of fluorine is very low. Indeed, they are the lowest-energy valence orbitals of ...
... The five 2p valence electrons of fluorine experience a highly positive effective nuclear charge of 5.2 and a Z2 very low n quantum number, so the value for eff is quite large. Thus, the energy of the valence orbitals n2 of fluorine is very low. Indeed, they are the lowest-energy valence orbitals of ...
Practice Packet Level 3: Atomics - Mr. Palermo`s Flipped Chemistry
... field. This suggested that cathode rays were composed of negatively charged particles found in all atoms. Thomson concluded that the atom was a positively charged sphere of almost uniform density in which ...
... field. This suggested that cathode rays were composed of negatively charged particles found in all atoms. Thomson concluded that the atom was a positively charged sphere of almost uniform density in which ...
A an electron and an alpha particle B an electron and a proton C a
... empty space ® electrons exist in orbitals outside the nucleus B the atom is a hard sphere ® electrons exist in orbitals outside the nucleus ® most of the atom is empty space C most of the atom is empty space ® electrons exist in orbitals outside the nucleus ® the atom is a hard sphere D most of the ...
... empty space ® electrons exist in orbitals outside the nucleus B the atom is a hard sphere ® electrons exist in orbitals outside the nucleus ® most of the atom is empty space C most of the atom is empty space ® electrons exist in orbitals outside the nucleus ® the atom is a hard sphere D most of the ...
General Chemistry I - University of Toledo
... 5.8 Calculate the wavelength of a moving object using the de Broglie equation. 5.9 Explain why the wavelength of macroscopic objects is not observed. 5.10 Calculate the uncertainty in the position of moving object if the velocity is known. 5.11 Identify and write valid sets of quantum numbers that d ...
... 5.8 Calculate the wavelength of a moving object using the de Broglie equation. 5.9 Explain why the wavelength of macroscopic objects is not observed. 5.10 Calculate the uncertainty in the position of moving object if the velocity is known. 5.11 Identify and write valid sets of quantum numbers that d ...