WRITING AP EQUATIONS AP equation sets are found in the free
... Active metals replace less active metals or hydrogen from their compounds in aqueous solution. Use an activity series or a reduction potential table to determine activity. The more easily oxidized metal replaces the less easily oxidized metal. The metal with the most negative reduction potential wi ...
... Active metals replace less active metals or hydrogen from their compounds in aqueous solution. Use an activity series or a reduction potential table to determine activity. The more easily oxidized metal replaces the less easily oxidized metal. The metal with the most negative reduction potential wi ...
Complete the following equations
... The concentration of Mg2+ in seawater is about 0.055 M. (a) How many liters of seawater will produce 1.00 kg of magnesium. (b) How many kilograms of calcium oxide, CaO, must be added to the seawater sample of part (a) in order to precipitate all of Mg2+ as magnesium hydroxide. (c) Write a balanced e ...
... The concentration of Mg2+ in seawater is about 0.055 M. (a) How many liters of seawater will produce 1.00 kg of magnesium. (b) How many kilograms of calcium oxide, CaO, must be added to the seawater sample of part (a) in order to precipitate all of Mg2+ as magnesium hydroxide. (c) Write a balanced e ...
Topic 8: ACIDS and BASES
... H3O+ or with a another base to form water. Other examples include CH3+ and Br+ (Hal+) which you will study in organic chemistry. molecules containing positive centres (mostly organic molecules) as a result of polar bonds within the molecule e.g. C in CO2 or in halogenalkanes and S in SO2. All Br ...
... H3O+ or with a another base to form water. Other examples include CH3+ and Br+ (Hal+) which you will study in organic chemistry. molecules containing positive centres (mostly organic molecules) as a result of polar bonds within the molecule e.g. C in CO2 or in halogenalkanes and S in SO2. All Br ...
Topic 8: ACIDS and BASES
... aqueous solutions, and therefore a higher pH, than strong acids of the same concentration. Strong acids: HCl, HNO3, H2SO4 , HBr, HI, H3PO4. Weak acids: CH3COOH (=ethanoic acid), H2CO3 (carbonic acid), HCOOH, citric acid, all carboxylic/organic acids. Exercise: For each of the above acids write an eq ...
... aqueous solutions, and therefore a higher pH, than strong acids of the same concentration. Strong acids: HCl, HNO3, H2SO4 , HBr, HI, H3PO4. Weak acids: CH3COOH (=ethanoic acid), H2CO3 (carbonic acid), HCOOH, citric acid, all carboxylic/organic acids. Exercise: For each of the above acids write an eq ...
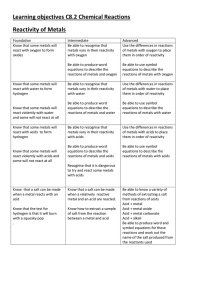
Learning objectives C8.2 Chemical Reactions Reactivity of Metals
... oxides form acid solutions when dissolved in and these cause acid rain Understand how the greenhouse effect works Understand why the level of CO2 in the atmosphere has increased in recent times due to burning of fossil fuels ...
... oxides form acid solutions when dissolved in and these cause acid rain Understand how the greenhouse effect works Understand why the level of CO2 in the atmosphere has increased in recent times due to burning of fossil fuels ...
The Elements of Group 15 (5A, V, VA) The Nitrogen Group
... Phosphorus is a tetrameric solid (white phosphorus) in its standard state (P4(s)), although it exists as many allotropes. White phosphorus reacts with oxygen (combusts), so must be stored under water. Formerly used in matches. ...
... Phosphorus is a tetrameric solid (white phosphorus) in its standard state (P4(s)), although it exists as many allotropes. White phosphorus reacts with oxygen (combusts), so must be stored under water. Formerly used in matches. ...
2013-2014
... If the reaction was complete in 2.5 minutes, which of the following, when plotted against time, would give a graph like the one shown above? A. Total mass of the conical flask and its contents ...
... If the reaction was complete in 2.5 minutes, which of the following, when plotted against time, would give a graph like the one shown above? A. Total mass of the conical flask and its contents ...
Introduction - Bulgarian Chemical Communications
... alternate conformation with equatorial 5-methyl will give rise to the strong repulsion between axial 6methyl and axial OH at 4-C, see Scheme 2, R4↔OH). 3-N is positively charged because of rate determining ureido group attack [21] and solvated respectively causing strong steric repulsion with an axi ...
... alternate conformation with equatorial 5-methyl will give rise to the strong repulsion between axial 6methyl and axial OH at 4-C, see Scheme 2, R4↔OH). 3-N is positively charged because of rate determining ureido group attack [21] and solvated respectively causing strong steric repulsion with an axi ...
4 • Reactions In Aqueous Solution
... equation for the reaction of washing soda, Na2CO3 and vinegar, HC2H3O2. ...
... equation for the reaction of washing soda, Na2CO3 and vinegar, HC2H3O2. ...
Document
... Some active metals such as sodium and calcium will react with water to give a metallic hydroxide and hydrogen gas. Ex: Ca + 2H2O Ca(OH)2 + H2 ...
... Some active metals such as sodium and calcium will react with water to give a metallic hydroxide and hydrogen gas. Ex: Ca + 2H2O Ca(OH)2 + H2 ...
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions (low pH). It can have harmful effects on plants, aquatic animals and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids. Governments have made efforts since the 1970s to reduce the release of sulfur dioxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes and sulfur dioxide is produced by volcanic eruptions. The chemicals in acid rain can cause paint to peel, corrosion of steel structures such as bridges, and erosion of stone statues.