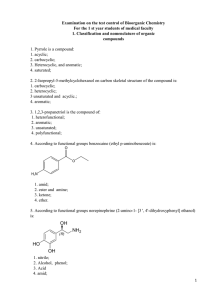
File - Mc Guckin Science
... 4. Find the percentage by mass of nitrogen in Al(NO 3)3. (19.7%) 5. Calculate the mass of 0.0250 mol of NaF. (1.05 g) 6. Calculate the mass of 1.50 L of argon gas, Ar, at STP. (2.67g) 7. A sample of a chemical was analyzed and found to contain 138 grams of sodium, 36 grams of carbon and 144 grams o ...
... 4. Find the percentage by mass of nitrogen in Al(NO 3)3. (19.7%) 5. Calculate the mass of 0.0250 mol of NaF. (1.05 g) 6. Calculate the mass of 1.50 L of argon gas, Ar, at STP. (2.67g) 7. A sample of a chemical was analyzed and found to contain 138 grams of sodium, 36 grams of carbon and 144 grams o ...
File - Varsity Field
... determined by subtracting the masses of C and H from the original compound mass. • Q1. Hexachlorophene, a compound made up of carbon, hydrogen, chlorine and oxygen atoms, is an ingredient used in germicidal soaps. Combustion of a 1.000 g sample of hexachlorophene yields 1.407 g CO2, 0.1340 g H2O and ...
... determined by subtracting the masses of C and H from the original compound mass. • Q1. Hexachlorophene, a compound made up of carbon, hydrogen, chlorine and oxygen atoms, is an ingredient used in germicidal soaps. Combustion of a 1.000 g sample of hexachlorophene yields 1.407 g CO2, 0.1340 g H2O and ...
Chemistry - SchoolNotes.com
... 15) If 20 g of magnesium reacts with excess hydrochloric acid (HCl), how many grams of magnesium chloride are produced? Mg + HCl --- MgCl2 + H2 78 g 16) How many grams of Na are required to react completely with 75.0 grams of chlorine using this reaction: 2 Na + Cl2 ---> 2 NaCl 49 g Na 17) Given th ...
... 15) If 20 g of magnesium reacts with excess hydrochloric acid (HCl), how many grams of magnesium chloride are produced? Mg + HCl --- MgCl2 + H2 78 g 16) How many grams of Na are required to react completely with 75.0 grams of chlorine using this reaction: 2 Na + Cl2 ---> 2 NaCl 49 g Na 17) Given th ...
chemistry advanced may 2010 marking scheme
... (d) Consider the following hydrides: CH4, NH3 and H2S. (i) State and explain briefly the acid–base properties (if any) of the substances. CH4 is neutral since it does not accept or donate protons. (0.5) NH3 is a base since it accepts protons to form NH4+. (0.5) H2S is an acid donating a proton to f ...
... (d) Consider the following hydrides: CH4, NH3 and H2S. (i) State and explain briefly the acid–base properties (if any) of the substances. CH4 is neutral since it does not accept or donate protons. (0.5) NH3 is a base since it accepts protons to form NH4+. (0.5) H2S is an acid donating a proton to f ...
1. Natures Chemistry Unit Questions
... In the reaction, the carbon atom next to the carbonyl functional group of one molecule forms a bond with the carbonyl carbon atom of the second molecule. (a) Draw a structural formula for the product formed when propanone is used instead of ethanal in this type of reaction. (1) (b) Name an aldehyde ...
... In the reaction, the carbon atom next to the carbonyl functional group of one molecule forms a bond with the carbonyl carbon atom of the second molecule. (a) Draw a structural formula for the product formed when propanone is used instead of ethanal in this type of reaction. (1) (b) Name an aldehyde ...
Exercise #5_Chpt 2
... Copper (II) sulfate is found as a hydrated salt, CuSO4.xH2O. A technician carefully heats 2.50g of the salt to a constant mass of 1.60g. a) What is meant by constant mass? b) How many moles of copper sulfate are there in 1.60g of anhydrous copper (II) sulfate? c) How many moles of water were lost? ...
... Copper (II) sulfate is found as a hydrated salt, CuSO4.xH2O. A technician carefully heats 2.50g of the salt to a constant mass of 1.60g. a) What is meant by constant mass? b) How many moles of copper sulfate are there in 1.60g of anhydrous copper (II) sulfate? c) How many moles of water were lost? ...
CHEMISTRY 110 LECTURE
... 2. A crucial reaction for the maintenance of plant and animal life is the conversion of oxygen gas to ozone gas[O3(g)] in the lower part of the stratosphere. How many molecules of oxygen gas are needed to produce 17.0 moles of ozone (O 3)? ...
... 2. A crucial reaction for the maintenance of plant and animal life is the conversion of oxygen gas to ozone gas[O3(g)] in the lower part of the stratosphere. How many molecules of oxygen gas are needed to produce 17.0 moles of ozone (O 3)? ...
REACTION PREDICTION
... All except Silver, Lead, Mercury I. Then you have sulfates, except for these three: Barium, Calcium and Lead, you see. Worry not only few left to go still. We will do fine on this test. Yes, we will! Then you have the--Insolubles Hydroxide, Sulfide and Carbonate and Phosphate, And all of these can b ...
... All except Silver, Lead, Mercury I. Then you have sulfates, except for these three: Barium, Calcium and Lead, you see. Worry not only few left to go still. We will do fine on this test. Yes, we will! Then you have the--Insolubles Hydroxide, Sulfide and Carbonate and Phosphate, And all of these can b ...
Chapter 6
... • Develop the reaction equation • Balance the reaction equation • Predict the state of matter of each species ...
... • Develop the reaction equation • Balance the reaction equation • Predict the state of matter of each species ...
Air pollution
... by coal-burning power plant and motor vehicle emissions, and in some regions, threatens human health, aquatic life and ecosystems, forests, and human-built structures. ...
... by coal-burning power plant and motor vehicle emissions, and in some regions, threatens human health, aquatic life and ecosystems, forests, and human-built structures. ...
Pollution ppt
... by coal-burning power plant and motor vehicle emissions, and in some regions, threatens human health, aquatic life and ecosystems, forests, and human-built structures. ...
... by coal-burning power plant and motor vehicle emissions, and in some regions, threatens human health, aquatic life and ecosystems, forests, and human-built structures. ...
Lab 6
... The analysis and identification of unknown organic compounds constitutes a very important aspect of experimental organic chemistry. There is no definite set procedure that can be applied overall to organic qualitative analysis. Some basic experimental tests and physical constants are necessary for i ...
... The analysis and identification of unknown organic compounds constitutes a very important aspect of experimental organic chemistry. There is no definite set procedure that can be applied overall to organic qualitative analysis. Some basic experimental tests and physical constants are necessary for i ...
CHEMICAL REACTIONS
... • e.g. Br2 + CH2=CH2 Æ CH2Br-CH2Br • e.g. H2 + CH2=CH2 Æ CH3-CH3 2. Substitution reactions Occur when an atom attached to carbon is replaced by something else. • e.g. Cl2 + CH4 Æ CH3Cl + HCl 3. Combustion Reactions When an organic compound is oxidized. Complete combustion results in carbon dioxide a ...
... • e.g. Br2 + CH2=CH2 Æ CH2Br-CH2Br • e.g. H2 + CH2=CH2 Æ CH3-CH3 2. Substitution reactions Occur when an atom attached to carbon is replaced by something else. • e.g. Cl2 + CH4 Æ CH3Cl + HCl 3. Combustion Reactions When an organic compound is oxidized. Complete combustion results in carbon dioxide a ...
Gas-forming Reactions
... Additionally the production of a volatile binary acid. These acids are in the gas phase and bubble out of the solution. H2S(g) and HCl(g) are common examples. ...
... Additionally the production of a volatile binary acid. These acids are in the gas phase and bubble out of the solution. H2S(g) and HCl(g) are common examples. ...
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions (low pH). It can have harmful effects on plants, aquatic animals and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids. Governments have made efforts since the 1970s to reduce the release of sulfur dioxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes and sulfur dioxide is produced by volcanic eruptions. The chemicals in acid rain can cause paint to peel, corrosion of steel structures such as bridges, and erosion of stone statues.