9. Entropy 2nd and 3rd laws/ Thermodynamic processes / Droplet
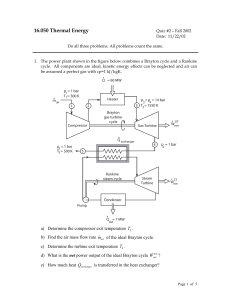
... 9.1 Entropy in second and third laws of thermodynamics (2pts) 1. Explain the statistical definition of entropy (4pts) 2. Consider a “thermodynamic system” of two dices and let the energy of a certain throw (state of the system) be the sum of the two values of the dices. Calculate the respective entr ...
... 9.1 Entropy in second and third laws of thermodynamics (2pts) 1. Explain the statistical definition of entropy (4pts) 2. Consider a “thermodynamic system” of two dices and let the energy of a certain throw (state of the system) be the sum of the two values of the dices. Calculate the respective entr ...
1-3 - University of Reading
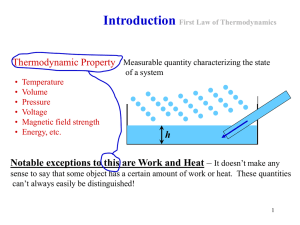
... T increases and or P (or density) decreases. • It has been experimentally confirmed. • PV = nkT is an example of an equation of state. P, V and T are state variables or thermodynamic coordinates. • Other equations of states can be defined to fit nonideal gas behaviour for example: van der Waals equa ...
... T increases and or P (or density) decreases. • It has been experimentally confirmed. • PV = nkT is an example of an equation of state. P, V and T are state variables or thermodynamic coordinates. • Other equations of states can be defined to fit nonideal gas behaviour for example: van der Waals equa ...
Solving Systems Using Elimination Warm Up: Notes:
... What two methods have we learned so far to solve system of equations? ...
... What two methods have we learned so far to solve system of equations? ...