8 Aromatic Compounds GOB Structures
... they are usually more dense than other hydrocarbons. • Halogenated benzene compounds are denser than water. • Aromatic hydrocarbons are insoluble in water and are used as solvents for other organic compounds. • Only aromatic compounds containing strongly polar functional groups such as — OH or — COO ...
... they are usually more dense than other hydrocarbons. • Halogenated benzene compounds are denser than water. • Aromatic hydrocarbons are insoluble in water and are used as solvents for other organic compounds. • Only aromatic compounds containing strongly polar functional groups such as — OH or — COO ...
Chem 400 Review Chem 350 JJ.S17
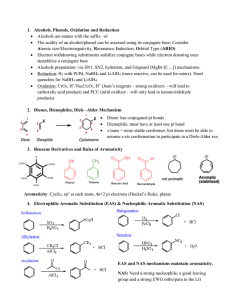
... Reduction: H2 with Pt/Pd, NaBH4 and LiAlH4 (more reactive, can be used for esters). Need quenches for NaBH4 and LiAlH4 Oxidation: CrO3, H+/Na2Cr2O7, H+ (Joan’s reagents – strong oxidizers – will lead to carboxylic acid product) and PCC (mild oxidizer – will only lead to ketone/aldehyde products) ...
... Reduction: H2 with Pt/Pd, NaBH4 and LiAlH4 (more reactive, can be used for esters). Need quenches for NaBH4 and LiAlH4 Oxidation: CrO3, H+/Na2Cr2O7, H+ (Joan’s reagents – strong oxidizers – will lead to carboxylic acid product) and PCC (mild oxidizer – will only lead to ketone/aldehyde products) ...
Uses and Sources of some Organic Molecules C11-5-14
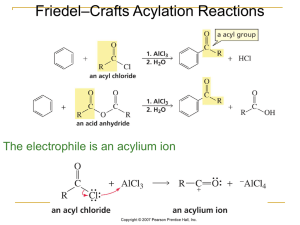
... are others as well but this activity will be limited to these types of hydrocarbon derivatives. Aromatic Hydrocarbons An aromatic hydrocarbon is a hydrocarbon in which the molecular structure includes one or more planar sets of six carbon atoms that are connected by delocalized electrons. (Delocaliz ...
... are others as well but this activity will be limited to these types of hydrocarbon derivatives. Aromatic Hydrocarbons An aromatic hydrocarbon is a hydrocarbon in which the molecular structure includes one or more planar sets of six carbon atoms that are connected by delocalized electrons. (Delocaliz ...
Mechanisms of organic reactions
... Substituents exhibiting the –M and – I effect (–CHO, –NO2) direct the next substituent to the meta position: ...
... Substituents exhibiting the –M and – I effect (–CHO, –NO2) direct the next substituent to the meta position: ...
Organic Chemistry - City University of New York
... • Although overlap of 2p orbitals occurs to form pi bonds, there is only minimal overlap between sets of 2p orbitals because they are not parallel. ...
... • Although overlap of 2p orbitals occurs to form pi bonds, there is only minimal overlap between sets of 2p orbitals because they are not parallel. ...
15. Benzene and Aromaticity
... Planar: bond angles are 120°, carbon–carbon bond lengths 139 pm Undergoes substitution rather than electrophilic addition Resonance hybrid with structure between two linebond structures Qualities similar for all Aromatic (4n+2) Compounds ...
... Planar: bond angles are 120°, carbon–carbon bond lengths 139 pm Undergoes substitution rather than electrophilic addition Resonance hybrid with structure between two linebond structures Qualities similar for all Aromatic (4n+2) Compounds ...