Atomic Structure PowerPoint Presentation
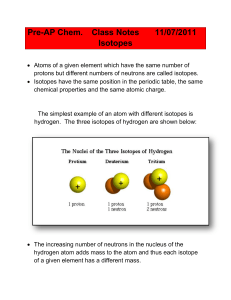
... o The atomic mass of an element represents the average mass of all the isotopes found in nature. No element exists with only one possible isotope. Hydrogen has the smallest number of isotopes: 1H protium, 2H deuterium, 3H tritium. Its atomic mass is 1.0079 amu (atomic mass units). The atomic mass is ...
... o The atomic mass of an element represents the average mass of all the isotopes found in nature. No element exists with only one possible isotope. Hydrogen has the smallest number of isotopes: 1H protium, 2H deuterium, 3H tritium. Its atomic mass is 1.0079 amu (atomic mass units). The atomic mass is ...
Element Project Part 2 PDF
... help us understand the behavior of atoms. Your model will be based on the Bohr model of the atom. Although the Bohr model of an atom is outdated, it still reinforces the concept that electrons a ...
... help us understand the behavior of atoms. Your model will be based on the Bohr model of the atom. Although the Bohr model of an atom is outdated, it still reinforces the concept that electrons a ...
Slide 1
... d) Formation of Ions When an electron is removed from an atom the overall charge will increase by +1 as an electron is -1 charged When an electron is added to an atom the overall charge will decrease by -1 as an electron is -1 charged This example shows the formation of a simple ionic crystal, sodi ...
... d) Formation of Ions When an electron is removed from an atom the overall charge will increase by +1 as an electron is -1 charged When an electron is added to an atom the overall charge will decrease by -1 as an electron is -1 charged This example shows the formation of a simple ionic crystal, sodi ...
Chapter 11
... repelled from each other! Something must serve as the glue to hold the nucleus together – The “strong” nuclear force: overcomes the electrical ...
... repelled from each other! Something must serve as the glue to hold the nucleus together – The “strong” nuclear force: overcomes the electrical ...
DEFINING THE ATOM - Southgate Schools
... ________ 11. The atomic number of an element is the sum of the protons and electrons in an atom of that element. ________ 12. The atomic number of an atom is the total number of protons in an atom of that element. ________ 13. An atom of nitrogen has 7 protons and 7 neutrons. ________ 14. Relative a ...
... ________ 11. The atomic number of an element is the sum of the protons and electrons in an atom of that element. ________ 12. The atomic number of an atom is the total number of protons in an atom of that element. ________ 13. An atom of nitrogen has 7 protons and 7 neutrons. ________ 14. Relative a ...
atoms - schultz915
... Democritus (400 B.C. ) – coins the term “atom” saying matter can be subdivided only as small as an elemental particle. from Greek “a” means not and “tomos” means cutting atom means “indivisible” ...
... Democritus (400 B.C. ) – coins the term “atom” saying matter can be subdivided only as small as an elemental particle. from Greek “a” means not and “tomos” means cutting atom means “indivisible” ...
atoms - schultz915
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
Atomic Structures Part
... Atomic Number and Protons Examples of atomic number and number of protons: ...
... Atomic Number and Protons Examples of atomic number and number of protons: ...
Atomic Structure PowerPoint
... there must be a quantum leap in energy. Schrodinger derived an equation that described the energy and position of the electrons in an atom ...
... there must be a quantum leap in energy. Schrodinger derived an equation that described the energy and position of the electrons in an atom ...
Naming Atoms — Elements, Ions and Isotopes
... Though electrons have the same, but opposite, charge as that of protons, their mass is extremely small compared to the protons and neutrons, which are approximately of equal mass. The mass of the atom is therefore determined by adding together the masses of the protons and neutrons. The total numbe ...
... Though electrons have the same, but opposite, charge as that of protons, their mass is extremely small compared to the protons and neutrons, which are approximately of equal mass. The mass of the atom is therefore determined by adding together the masses of the protons and neutrons. The total numbe ...
Atomic Mass - AJS Phyiscs and Chemistry
... • First person to discover a sub-atomic particle (a particle smaller than an atom). • Thomson conducted a series of experiments with cathode ray tubes leading him to the discovery of electrons. • He proposed that the atom contained a positively charged sphere in which negatively charged electrons we ...
... • First person to discover a sub-atomic particle (a particle smaller than an atom). • Thomson conducted a series of experiments with cathode ray tubes leading him to the discovery of electrons. • He proposed that the atom contained a positively charged sphere in which negatively charged electrons we ...
Ch # 5 Notes
... Atom: An atom is the smallest particle of an element that can exist and still have properties of the element. Atomic Theory of matter: 1) All matter is made up of small particles called atoms.113 types. 2) Atoms of same element are similar to one another. 3) The relative number and arrangement of di ...
... Atom: An atom is the smallest particle of an element that can exist and still have properties of the element. Atomic Theory of matter: 1) All matter is made up of small particles called atoms.113 types. 2) Atoms of same element are similar to one another. 3) The relative number and arrangement of di ...
Neutron Number (N = AZ) = # Neutrons
... have enough energy to knock electrons out of the atoms, so a current can flow. This is the basis of solar energy. This process requires a certain minimum energy, known as the work function . So if a photon E hf strikes a photoelectric material some of its energy is required to move the electron c ...
... have enough energy to knock electrons out of the atoms, so a current can flow. This is the basis of solar energy. This process requires a certain minimum energy, known as the work function . So if a photon E hf strikes a photoelectric material some of its energy is required to move the electron c ...
By 1911 the components of the atom had been discovered
... ● If Thomson's Plum Pudding Model was correct, the positive charge of the atom is spread out over a relatively large area. ○ this would mean that the effect of this positive charge would be very weak on other charges, because it is so spread out. ○ the negative electrons are unimportant since they a ...
... ● If Thomson's Plum Pudding Model was correct, the positive charge of the atom is spread out over a relatively large area. ○ this would mean that the effect of this positive charge would be very weak on other charges, because it is so spread out. ○ the negative electrons are unimportant since they a ...
Isotope Practice Worksheet
... Some isotopes are stable while others are unstable or radioactive. Radioactive isotopes emit nuclear radiation in the form of rapidly moving particles or high energy electromagnetic waves. The particles are emitted from the nucleus itself and their removal results in changing the atom from one ...
... Some isotopes are stable while others are unstable or radioactive. Radioactive isotopes emit nuclear radiation in the form of rapidly moving particles or high energy electromagnetic waves. The particles are emitted from the nucleus itself and their removal results in changing the atom from one ...
STAAR Science Tutorial 09 TEK 8.5A: Atomic Structure
... Electrons have a negative (-) charge, and a very tiny mass of 0.005 amu. The mass of electrons are so small that they are usually ignored when adding up the mass of the entire atom to state the atom’s atomic mass. ...
... Electrons have a negative (-) charge, and a very tiny mass of 0.005 amu. The mass of electrons are so small that they are usually ignored when adding up the mass of the entire atom to state the atom’s atomic mass. ...
File - Flipped Out Science with Mrs. Thomas!
... Electrons have a negative (-) charge, and a very tiny mass of 0.005 amu. The mass of electrons are so small that they are usually ignored when adding up the mass of the entire atom to state the atom’s atomic mass. ...
... Electrons have a negative (-) charge, and a very tiny mass of 0.005 amu. The mass of electrons are so small that they are usually ignored when adding up the mass of the entire atom to state the atom’s atomic mass. ...
TEK 8.5A: Atomic Structure
... Electrons have a negative (-) charge, and a very tiny mass of 0.0005 amu. The mass of electrons are so small that they are usually ignored when adding up the mass of the entire atom to state the atom’s atomic mass. ...
... Electrons have a negative (-) charge, and a very tiny mass of 0.0005 amu. The mass of electrons are so small that they are usually ignored when adding up the mass of the entire atom to state the atom’s atomic mass. ...
chapter 5 Radioactivity
... nucleus, the electron created in the process could not exist inside the nucleus and therefore must be ejected. Iodine-131 undergoes this type of beta decay: I ...
... nucleus, the electron created in the process could not exist inside the nucleus and therefore must be ejected. Iodine-131 undergoes this type of beta decay: I ...
Static electricity
... Particle name Relative Mass Relative (how their masses compare charge with each other) Neutron ...
... Particle name Relative Mass Relative (how their masses compare charge with each other) Neutron ...
File
... Through gold foil experiment, discovered the presence of concentrated positive charge in the core of an atom. (Proton and nucleus) Electrons circulate around the nucleus of an atom within a specific electron shell. ...
... Through gold foil experiment, discovered the presence of concentrated positive charge in the core of an atom. (Proton and nucleus) Electrons circulate around the nucleus of an atom within a specific electron shell. ...
Beta decay is a type of radioactive decay in which a beta
... Beta decay does not change the number of nucleons, A, in the nucleus; it changes only its charge, Z. Therefore the set of allnuclides with the same A can be introduced; these isobaric nuclides may turn into each other via beta decay. A beta-stable nucleus may undergo other kinds of radioactive decay ...
... Beta decay does not change the number of nucleons, A, in the nucleus; it changes only its charge, Z. Therefore the set of allnuclides with the same A can be introduced; these isobaric nuclides may turn into each other via beta decay. A beta-stable nucleus may undergo other kinds of radioactive decay ...
File - Science With BLT
... 5. How do the charge and mass of an electron compare to the charge and mass of a proton? a. An electron has the opposite charge and a smaller mass than a proton. b. An electron has the opposite charge and the same mass as a proton. c. An electron has the same charge and the same mass as a proton. d. ...
... 5. How do the charge and mass of an electron compare to the charge and mass of a proton? a. An electron has the opposite charge and a smaller mass than a proton. b. An electron has the opposite charge and the same mass as a proton. c. An electron has the same charge and the same mass as a proton. d. ...
PowerPoint - Models of the Atom
... So, atoms are mostly empty. Some positive -particles deflected or bounced back! Thus, a “nucleus” is positive & holds most of an atom’s mass. ...
... So, atoms are mostly empty. Some positive -particles deflected or bounced back! Thus, a “nucleus” is positive & holds most of an atom’s mass. ...