CHEM 250Q
... Each of the elements in the periodic table has a specific number. What does this number represent? A. ...
... Each of the elements in the periodic table has a specific number. What does this number represent? A. ...
physical setting chemistry
... the instructions from the proctor for completing the student information on your answer sheet. Record your answers to the Part A and Part B–1 multiple-choice questions on this separate answer sheet. Record your answers for the questions in Part B–2 and Part C in your separate answer booklet. Be sure ...
... the instructions from the proctor for completing the student information on your answer sheet. Record your answers to the Part A and Part B–1 multiple-choice questions on this separate answer sheet. Record your answers for the questions in Part B–2 and Part C in your separate answer booklet. Be sure ...
Chapter 3 Molecules, Compounds, and Chemical Equations q
... - a compound is a distinct substance that is composed of atoms of two or more elements. - describe the compound by describing the number and type of each atom in the simplest unit of the compound. molecules or ions - each element is represented by its letter symbol. - the number of atoms of each e ...
... - a compound is a distinct substance that is composed of atoms of two or more elements. - describe the compound by describing the number and type of each atom in the simplest unit of the compound. molecules or ions - each element is represented by its letter symbol. - the number of atoms of each e ...
CHAPTER 1
... any substance that has a definite composition. For example, consider the material called sucrose, or cane sugar. It has a definite composition in terms of the atoms that compose it. It is produced by certain plants in the chemical process of photosynthesis. Sucrose is a chemical. Carbon dioxide, wat ...
... any substance that has a definite composition. For example, consider the material called sucrose, or cane sugar. It has a definite composition in terms of the atoms that compose it. It is produced by certain plants in the chemical process of photosynthesis. Sucrose is a chemical. Carbon dioxide, wat ...
Option C Energy - Cambridge Resources for the IB Diploma
... A typical family car could have an average fuel consumption of 7 litres (dm3) of gasoline per 100 km – so to travel the same distance more than 1500 litres (dm3) of hydrogen gas would be required (the size of a trunk/ boot of a car is typically about 400 dm3). One solution to this problem would be t ...
... A typical family car could have an average fuel consumption of 7 litres (dm3) of gasoline per 100 km – so to travel the same distance more than 1500 litres (dm3) of hydrogen gas would be required (the size of a trunk/ boot of a car is typically about 400 dm3). One solution to this problem would be t ...
Equilibrium Electrochemistry
... formation of ATP in the cell). Glycolysis is the main source of energy during anaerobic metabolism, a form of metabolism in which inhaled O2 does not play a role. The citric acid cycle & oxidative phosphorylation are the main mechanisms for the extraction of energy from carbohydrates during aero ...
... formation of ATP in the cell). Glycolysis is the main source of energy during anaerobic metabolism, a form of metabolism in which inhaled O2 does not play a role. The citric acid cycle & oxidative phosphorylation are the main mechanisms for the extraction of energy from carbohydrates during aero ...
Chemical Reactivity as Described by Quantum Chemical Methods
... "modern" quantum mechanics around 1925 due to the work of Schrödinger, Heisenberg, Born, … Schrödinger's equation occupied a central position in this new theory and, although later on complemented by its relativistic analogue by Dirac, stood the test of time and has been for now 75 years the central ...
... "modern" quantum mechanics around 1925 due to the work of Schrödinger, Heisenberg, Born, … Schrödinger's equation occupied a central position in this new theory and, although later on complemented by its relativistic analogue by Dirac, stood the test of time and has been for now 75 years the central ...
Fundamentals of Chemistry
... atom is an extremely small electrically-neutral particle. It is the smallest unit involved in the chemical change of matter. Atoms can be treated as distinct particles because they behave as such chemically, but atoms themselves are composed of even smaller subparts. Understanding these atomic subpa ...
... atom is an extremely small electrically-neutral particle. It is the smallest unit involved in the chemical change of matter. Atoms can be treated as distinct particles because they behave as such chemically, but atoms themselves are composed of even smaller subparts. Understanding these atomic subpa ...
Midterm Practice Exam Key
... Aqueous Reactions (5 marks) 1. A substance is considered ____________ if it will dissolve in a specific solvent. 2. An ____________ in the oxidation number of an atom signifies oxidation, while a ____________ in the oxidation number signifies reduction. 3. A ____________ reaction is one in which ...
... Aqueous Reactions (5 marks) 1. A substance is considered ____________ if it will dissolve in a specific solvent. 2. An ____________ in the oxidation number of an atom signifies oxidation, while a ____________ in the oxidation number signifies reduction. 3. A ____________ reaction is one in which ...
ordinary level chemistry syllabus
... the essential practical skills and competencies a learner should acquire during and at the end of each unit of learning. 1.2. Rationale of teaching and learning of chemistry 1.2.1. Chemistry and society Chemistry, one of the natural science subjects, is an important discipline that has contributed s ...
... the essential practical skills and competencies a learner should acquire during and at the end of each unit of learning. 1.2. Rationale of teaching and learning of chemistry 1.2.1. Chemistry and society Chemistry, one of the natural science subjects, is an important discipline that has contributed s ...
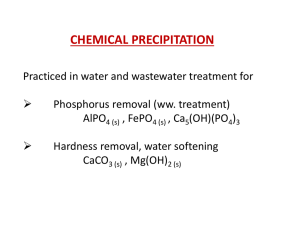
Phosphorus Removal from Wastewater by Chemical Precipitation
... • Lime is cheaper than alum, FeCl3 or Fe2(SO4)3. • It can achieve the lowest residual phosphate concentration because of the formation of apatites, Ca5(OH)(PO4)3. • Apatites are highly insoluble Ksp = 10-55.0, however a high pH must be attained to realize their formation. • Because calcium forms CaC ...
... • Lime is cheaper than alum, FeCl3 or Fe2(SO4)3. • It can achieve the lowest residual phosphate concentration because of the formation of apatites, Ca5(OH)(PO4)3. • Apatites are highly insoluble Ksp = 10-55.0, however a high pH must be attained to realize their formation. • Because calcium forms CaC ...
CHEMISTRY CHM-050 Introduction to Chemistry I NCC Cr: 3 D Lec
... Prerequisite: MAT-063, Elementary Algebra, or equivalent. A onesemester college chemistry course which surveys important concepts and topics of chemistry. Among these are the metric system of measurement, atomic theory of matter, energy levels and atomic structure, the periodic table, ionic and mole ...
... Prerequisite: MAT-063, Elementary Algebra, or equivalent. A onesemester college chemistry course which surveys important concepts and topics of chemistry. Among these are the metric system of measurement, atomic theory of matter, energy levels and atomic structure, the periodic table, ionic and mole ...
Task 4 6 points - Austrian Chemistry Olympiad
... The Austrian company Sandoz GmbH with their factory in Tirol is the largest producer of antibiotics and the only real producer of penicillin in the western world. The company accounts for approximately two-thirds of the worldwide production of penicillin V, the acid stable penicillin that was discov ...
... The Austrian company Sandoz GmbH with their factory in Tirol is the largest producer of antibiotics and the only real producer of penicillin in the western world. The company accounts for approximately two-thirds of the worldwide production of penicillin V, the acid stable penicillin that was discov ...