Scanning Tunneling Microscope
... The best results from STM can be obtained only in vacuum conditions,hence it may not be the best tool to inspect and analyse biological samples Damaged tip[6] ...
... The best results from STM can be obtained only in vacuum conditions,hence it may not be the best tool to inspect and analyse biological samples Damaged tip[6] ...
Scanning Tunneling Microscope
... The best results from STM can be obtained only in vacuum conditions,hence it may not be the best tool to inspect and analyse biological samples Damaged tip[6] ...
... The best results from STM can be obtained only in vacuum conditions,hence it may not be the best tool to inspect and analyse biological samples Damaged tip[6] ...
Communicating chemistry for public engagement
... With the structure being a chemist’s primary form of communication, how can the non-chemist be expected to understand? The field is also marked by complexity. And although chemists and those who admire chemistry may find beauty in this complexity, it is easy to see why some non-chemists find the sci ...
... With the structure being a chemist’s primary form of communication, how can the non-chemist be expected to understand? The field is also marked by complexity. And although chemists and those who admire chemistry may find beauty in this complexity, it is easy to see why some non-chemists find the sci ...
AP CHEMISTRY
... 8. Calculate the percent error that resulted if the theoretically accepted value (according to the handbook of Chemistry & Physics) for the sample measured is known to be 0.703 g/cm3. ...
... 8. Calculate the percent error that resulted if the theoretically accepted value (according to the handbook of Chemistry & Physics) for the sample measured is known to be 0.703 g/cm3. ...
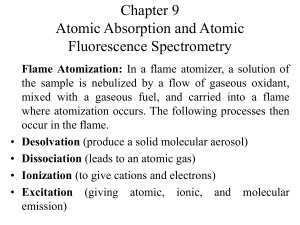
Chapter 9 Atomic Absorption and Atomic Fluorescence Spectrometry
... average residence time of the atoms in the optical path is a second or more. A few microliters of sample are first evaporated at a low temperature and then ashed at a somewhat higher temperature in an electrically heated graphite tube or in a graphite cup. Then the current is rapidly increased to se ...
... average residence time of the atoms in the optical path is a second or more. A few microliters of sample are first evaporated at a low temperature and then ashed at a somewhat higher temperature in an electrically heated graphite tube or in a graphite cup. Then the current is rapidly increased to se ...
Chemistry
... Part I Qualitative Analysis Inorganic mixtures containing five radicals/ions to be prepared from the following list: Ag+, Pb2+, Hg22+, Hg2+, Bi3+, Cu2+, Cd2+, As3+, Sb3+, Sn2+, Sn4+, Fe3+, Al3+, Ba2+, Cr3+, Zn2+, Mn2+, Co2+, Ni2+, Ca2+, Sr2+, Mg2+: , Cl-, Br-, I-, SO42-, SO32-, S2-, CrO42-, PO43-, N ...
... Part I Qualitative Analysis Inorganic mixtures containing five radicals/ions to be prepared from the following list: Ag+, Pb2+, Hg22+, Hg2+, Bi3+, Cu2+, Cd2+, As3+, Sb3+, Sn2+, Sn4+, Fe3+, Al3+, Ba2+, Cr3+, Zn2+, Mn2+, Co2+, Ni2+, Ca2+, Sr2+, Mg2+: , Cl-, Br-, I-, SO42-, SO32-, S2-, CrO42-, PO43-, N ...
AP Chemistry Summer Assignment
... 38.Strontium consists of four isotopes with masses and their percent abundance of 83.9134 amu ( 0.5%), 85.9094 amu (9.9%) , 86.9089 amu (7.0 %) , and 87.9056 amu (82.6 %). Calculate the atomic mass of Sr ? 39.Nitrogen has two isotopes, N-14 and N-15, with atomic masses of 14.00031 amu and 15.001 amu ...
... 38.Strontium consists of four isotopes with masses and their percent abundance of 83.9134 amu ( 0.5%), 85.9094 amu (9.9%) , 86.9089 amu (7.0 %) , and 87.9056 amu (82.6 %). Calculate the atomic mass of Sr ? 39.Nitrogen has two isotopes, N-14 and N-15, with atomic masses of 14.00031 amu and 15.001 amu ...
Philosophy of Chemistry
... mechanical approaches were among several competing approaches within theoretical chemistry, though not very successful. The question became meaningful only with the development of quantum mechanics and its application to chemistry since the late 1920s. Following a speech by Paul Dirac in 1929, many ...
... mechanical approaches were among several competing approaches within theoretical chemistry, though not very successful. The question became meaningful only with the development of quantum mechanics and its application to chemistry since the late 1920s. Following a speech by Paul Dirac in 1929, many ...
Chemistry B – Introduction to Chemical Reactions
... student discussion, and hands-on activities. When appropriate and possible examples from engineering, material science, medicine, the environment, and our everyday experience will be used for chemistry concepts. This course is merely an introduction to the concepts and mathematics of chemistry. Do n ...
... student discussion, and hands-on activities. When appropriate and possible examples from engineering, material science, medicine, the environment, and our everyday experience will be used for chemistry concepts. This course is merely an introduction to the concepts and mathematics of chemistry. Do n ...
Yearly Plan for MYP 1 Science
... Name and give the formulas for some common compound ions Write the formula for simple compounds that include compound ions from their names Name simple compounds that include compound ions from their formulas Define and describe ionic and covalent bonds Name examples and describe general characteris ...
... Name and give the formulas for some common compound ions Write the formula for simple compounds that include compound ions from their names Name simple compounds that include compound ions from their formulas Define and describe ionic and covalent bonds Name examples and describe general characteris ...
DEPARTMENT OF CHEMISTRY Course Book for M.Sc. in Chemistry
... Objective: Quantum Mechanics is a branch of science that deals with discrete, indivisible units of energy called quanta as described by the Quantum. It is an interfacial subject between Physics, chemistry and mathematics. Hence the objective of this course in chemistry is to understand clearly the m ...
... Objective: Quantum Mechanics is a branch of science that deals with discrete, indivisible units of energy called quanta as described by the Quantum. It is an interfacial subject between Physics, chemistry and mathematics. Hence the objective of this course in chemistry is to understand clearly the m ...
Prescribed Practicals
... relatively high molar mass that allows for a high accuracy. A solution of KHP is used to determine the exact concentration of a solution like NaOH. NaOH cannot be used to make a standard solution because it is hygroscopic and unstable, therefore, it cannot be made to an exact concentration. Sodi ...
... relatively high molar mass that allows for a high accuracy. A solution of KHP is used to determine the exact concentration of a solution like NaOH. NaOH cannot be used to make a standard solution because it is hygroscopic and unstable, therefore, it cannot be made to an exact concentration. Sodi ...
Chemistry - Swami Ramanand Teerth Marathwada University
... against weak base. (iii) Weak acid against strong base. (iv) Weak acid against weak base. v) Precipitation titration. i) Advantages of conductometric titrations. j) Numericals on Specific conductance, Equivalent conductance, cell constant and Kohlrausch law to calculate λ . Unit III: ...
... against weak base. (iii) Weak acid against strong base. (iv) Weak acid against weak base. v) Precipitation titration. i) Advantages of conductometric titrations. j) Numericals on Specific conductance, Equivalent conductance, cell constant and Kohlrausch law to calculate λ . Unit III: ...
Symbol
... 16. Find the density of a block of wood with a mass of 25.00 g and a volume of 80.000 cm3 17. An irregularly shaped stone was lowered into a graduated cylinder holding a volume of water equal to 2 ml. The height of the water rose to 7 ml. If the mass of the stone was 25 g, what was its density? 18. ...
... 16. Find the density of a block of wood with a mass of 25.00 g and a volume of 80.000 cm3 17. An irregularly shaped stone was lowered into a graduated cylinder holding a volume of water equal to 2 ml. The height of the water rose to 7 ml. If the mass of the stone was 25 g, what was its density? 18. ...
Chapter 4 Power Point Quiz
... Ammonia is produced using the Haber process: 3 H2 + N2 2 NH3 What mass of ammonia could be produced from 15.0 kg each of H2 and N2? Assume the reaction goes to completion. a) b) c) d) e) ...
... Ammonia is produced using the Haber process: 3 H2 + N2 2 NH3 What mass of ammonia could be produced from 15.0 kg each of H2 and N2? Assume the reaction goes to completion. a) b) c) d) e) ...
VCAA Study Design - Chemistry Education Association
... Essential Learning Standards (VELS) - has been designed to ensure that schools and teachers are not required to manage two different curriculum and reporting frameworks during the development of the Australian Curriculum - uses an eleven level structure to reflect the design of the new Australian Cu ...
... Essential Learning Standards (VELS) - has been designed to ensure that schools and teachers are not required to manage two different curriculum and reporting frameworks during the development of the Australian Curriculum - uses an eleven level structure to reflect the design of the new Australian Cu ...
Department of Chemistry
... measurement of chemical properties, structures, and phenomena; hands-on experience with modern analytical instrumentation; and computational data analysis and modeling. All laboratory programs are conducted in a safe environment that includes adherence to national and state regulations regarding haz ...
... measurement of chemical properties, structures, and phenomena; hands-on experience with modern analytical instrumentation; and computational data analysis and modeling. All laboratory programs are conducted in a safe environment that includes adherence to national and state regulations regarding haz ...
Setting the stage
... Infrared spectroscopy utilized in chemical simulations of reactions on interstellar ices. Various studies have been performed on amphiphilic vesicle forming molecules. ...
... Infrared spectroscopy utilized in chemical simulations of reactions on interstellar ices. Various studies have been performed on amphiphilic vesicle forming molecules. ...
Intro to Titrimetry
... Precipitation Titration – forms insoluble salts which also serves as a monitor of reaction completion. Ex: Volhard Methods and Fajans Method RedOx Titrations – involves species which undergo redox reactions. The reaction is monitored by RedOx indicators or ...
... Precipitation Titration – forms insoluble salts which also serves as a monitor of reaction completion. Ex: Volhard Methods and Fajans Method RedOx Titrations – involves species which undergo redox reactions. The reaction is monitored by RedOx indicators or ...
Chapter 1 Reading Guide
... Guidelines for determining the number of significant figures in a measured quantity are: • The number of significant figures is the number of digits known with certainty plus one uncertain digit. (Example: 2.2405 g means we are sure the mass is 2.240 g but we are uncertain about the nearest 0.0001 g ...
... Guidelines for determining the number of significant figures in a measured quantity are: • The number of significant figures is the number of digits known with certainty plus one uncertain digit. (Example: 2.2405 g means we are sure the mass is 2.240 g but we are uncertain about the nearest 0.0001 g ...
Name ______Mr. Perfect_______________________________
... Name ______Mr. Perfect_______________________________ Date ____Sp 09_____ 1. If the n quantum number of an atomic orbital is equal to 4, what are the possible values of l ? What are the possible values of ml if the quantum number l is equal to 1? (5 pts) l ranges from 0 to n-1 ...
... Name ______Mr. Perfect_______________________________ Date ____Sp 09_____ 1. If the n quantum number of an atomic orbital is equal to 4, what are the possible values of l ? What are the possible values of ml if the quantum number l is equal to 1? (5 pts) l ranges from 0 to n-1 ...
preliminary course outline facilitators course description
... (e.g., take the things that you need before entering the classroom, quietly take/leave the seat without interrupting those around you). No cell phones or headphones in class. Browsing facebook, streaming sports, movies and playing games in class is very distracting to other class participants. Pleas ...
... (e.g., take the things that you need before entering the classroom, quietly take/leave the seat without interrupting those around you). No cell phones or headphones in class. Browsing facebook, streaming sports, movies and playing games in class is very distracting to other class participants. Pleas ...
CHEMISTRY Academic Standards Statement
... chemical bonds in a chemical species give rise to the shape, structure and microscopic properties of that species. 2.1.2 Methods of structure determination i. A variety of experimental (e.g. spectroscopic, spectrometric and diffraction) and theoretical methods can be used to determine molecular stru ...
... chemical bonds in a chemical species give rise to the shape, structure and microscopic properties of that species. 2.1.2 Methods of structure determination i. A variety of experimental (e.g. spectroscopic, spectrometric and diffraction) and theoretical methods can be used to determine molecular stru ...
Analytical chemistry

Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample, and quantitative analysis determines the amount of certain components in the substance. The separation of components is often performed prior to analysis.Analytical methods can be separated into classical and instrumental. Classical methods (also known as wet chemistry methods) use separations such as precipitation, extraction, and distillation and qualitative analysis by color, odor, or melting point. Classical quantitative analysis is achieved by measurement of weight or volume. Instrumental methods use an apparatus to measure physical quantities of the analyte such as light absorption, fluorescence, or conductivity. The separation of materials is accomplished using chromatography, electrophoresis or field flow fractionation methods.Analytical chemistry is also focused on improvements in experimental design, chemometrics, and the creation of new measurement tools to provide better chemical information. Analytical chemistry has applications in forensics, bioanalysis, clinical analysis, environmental analysis, and materials analysis.