Earth Chemistry
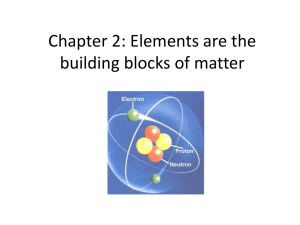
... • Electrons orbit the nucleus like planets orbiting the sun. • The orbits called electron shells or orbitals • close to the nucleus hold fewer electrons than those far away. ...
... • Electrons orbit the nucleus like planets orbiting the sun. • The orbits called electron shells or orbitals • close to the nucleus hold fewer electrons than those far away. ...
2 ppt
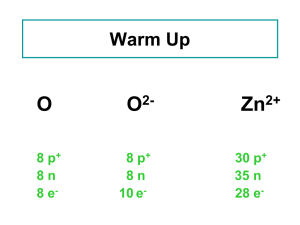
... The number of protons in an atom determines the element # of protons = atomic number this also tells you # of electrons ...
... The number of protons in an atom determines the element # of protons = atomic number this also tells you # of electrons ...
Getting to know and love our atoms, more and more each day
... 1st orbital : holds at most _______ electrons 2nd and 3rd orbital: holds at most _______ electrons 4th and beyond orbital: holds at most ________ electrons 6. How to draw a Bohr model 1) draw a nucleus (write inside the number of protons and neutrons) 2) draw the orbitals filling in the correct numb ...
... 1st orbital : holds at most _______ electrons 2nd and 3rd orbital: holds at most _______ electrons 4th and beyond orbital: holds at most ________ electrons 6. How to draw a Bohr model 1) draw a nucleus (write inside the number of protons and neutrons) 2) draw the orbitals filling in the correct numb ...
Chapter 2: Elements are the building blocks of matter
... • Organizes the elements according to their physical and chemical properties • The one we use was developed by Dmitri Mendeleev in 1867 ...
... • Organizes the elements according to their physical and chemical properties • The one we use was developed by Dmitri Mendeleev in 1867 ...
Exam Review - hrsbstaff.ednet.ns.ca
... The reaction of solutions of ammonium phosphate and barium nitrate gives a precipitate of barium phosphate. The equation that best represents this statement is a) 2(NH4)3PO4(s) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(s). b) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(s) + 6NH4NO3(aq). c) 2(NH4)3PO4 ...
... The reaction of solutions of ammonium phosphate and barium nitrate gives a precipitate of barium phosphate. The equation that best represents this statement is a) 2(NH4)3PO4(s) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(s). b) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(s) + 6NH4NO3(aq). c) 2(NH4)3PO4 ...
Ch 4 Review PowerPoint ch4jeopardy_review1
... Discovered that atoms contain a positive nucleus and are mostly empty space. ...
... Discovered that atoms contain a positive nucleus and are mostly empty space. ...
Chapter 4 Atomic Structure
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
Chapter 4 Atomic Structure
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
atoms - Harjono
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
atoms - Chemistry
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
Why are atoms of lead different to those of gold and why can we not
... turn lead into gold, but why can we not? Why are atoms of lead different to those of gold and why can we not just simply change them? ...
... turn lead into gold, but why can we not? Why are atoms of lead different to those of gold and why can we not just simply change them? ...
Chapter 3, Section One - Bismarck Public Schools
... –Atoms make up elements such as oxygen, nitrogen, and helium. •Check out your periodic table! –Atoms are the smallest part of an element that still has all the same properties of an element. What is an Atom? •Today, scientists believe the following about atoms…. •All elements are composed of atoms. ...
... –Atoms make up elements such as oxygen, nitrogen, and helium. •Check out your periodic table! –Atoms are the smallest part of an element that still has all the same properties of an element. What is an Atom? •Today, scientists believe the following about atoms…. •All elements are composed of atoms. ...
Isotopes-Chemistry
... Same Element Different AtomIsotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) ...
... Same Element Different AtomIsotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) ...
Chapter 2
... from charged atoms or groups of atoms called ions – Metal atoms tend to lose electrons: result is a positive charge (cation) – Nonmetals tend to gain electrons: result is a negative charge (anion) • Sodium loses one electron to become Na+ • Calcium loses two electrons to become Ca2+ • May consist of ...
... from charged atoms or groups of atoms called ions – Metal atoms tend to lose electrons: result is a positive charge (cation) – Nonmetals tend to gain electrons: result is a negative charge (anion) • Sodium loses one electron to become Na+ • Calcium loses two electrons to become Ca2+ • May consist of ...
Chapter 4 Section 4.3
... • So instead, we compare the relative masses of atoms using a reference isotope as a standard. • The reference isotope chosen is carbon-12. • The isotope of carbon has been assigned a mass of exactly 12 atomic mass units. ...
... • So instead, we compare the relative masses of atoms using a reference isotope as a standard. • The reference isotope chosen is carbon-12. • The isotope of carbon has been assigned a mass of exactly 12 atomic mass units. ...
Bohr Models - sci9sage-wmci
... Models- a way of showing/picturing how something works. Based on a Theory ...
... Models- a way of showing/picturing how something works. Based on a Theory ...
Early Greek Philosophers determined that atoms are the building
... Have properties of both metals and nonmetals Located on either side of the zigzag line separating metals and nonmetals Most common is Silicon ...
... Have properties of both metals and nonmetals Located on either side of the zigzag line separating metals and nonmetals Most common is Silicon ...
Chapter 4 Atomic Structure
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
Chemistry ppt - Plain Local Schools
... A. Describe that matter is made of minute particles called atoms and that atoms are comprised of even smaller components. Explain the structure and properties of atoms. B. Explain how atoms react with each other to form substances and how molecules react with each other or other atoms to form even d ...
... A. Describe that matter is made of minute particles called atoms and that atoms are comprised of even smaller components. Explain the structure and properties of atoms. B. Explain how atoms react with each other to form substances and how molecules react with each other or other atoms to form even d ...