Chapter 4
... 15. Another name for starting friction is static friction, it is the frictional force that is needed to budge a static or stationary object. If a power supply weighing 22Lb is to be slid across a table where the coefficient of starting friction is 0.5, how much force is needed to budge the supply? 1 ...
... 15. Another name for starting friction is static friction, it is the frictional force that is needed to budge a static or stationary object. If a power supply weighing 22Lb is to be slid across a table where the coefficient of starting friction is 0.5, how much force is needed to budge the supply? 1 ...
Forces 2014-15 v2 - McKinney ISD Staff Sites
... – Rubbing hands together – Pushing a heavy object over a rough surface – Pouring sand on ice ...
... – Rubbing hands together – Pushing a heavy object over a rough surface – Pouring sand on ice ...
Glossary: Unit I
... point of incidence. angle of reflection: the angle a reflected ray makes with the normal to the surface at the point of reflection. antinode: a point on a standing wave where the displacement of the medium is at its maximum. atom: the smallest particle of an element that has all the element’s proper ...
... point of incidence. angle of reflection: the angle a reflected ray makes with the normal to the surface at the point of reflection. antinode: a point on a standing wave where the displacement of the medium is at its maximum. atom: the smallest particle of an element that has all the element’s proper ...
1. Why must an object at rest have either no force or at least two
... 13. A gardener pushes down along the handle of a lawn mower of 20 kg mass with a force of 150 N. The handle makes an angle of 60° with the ground. Calculate the instantaneous acceleration of the mower if the frictional force between its wheels and the ground at that instant is 25 N. 14. A boy with ...
... 13. A gardener pushes down along the handle of a lawn mower of 20 kg mass with a force of 150 N. The handle makes an angle of 60° with the ground. Calculate the instantaneous acceleration of the mower if the frictional force between its wheels and the ground at that instant is 25 N. 14. A boy with ...
PHYS 1020 Lecture 18 Work Energy
... A common example of academic dishonesty that occurs every term: • A student brings a calculator to the final exam. The calculator is allowed by the instructor, but the calculator cover is forbidden. • The student forgets that the cover is still attached to the calculator and that his/her study list ...
... A common example of academic dishonesty that occurs every term: • A student brings a calculator to the final exam. The calculator is allowed by the instructor, but the calculator cover is forbidden. • The student forgets that the cover is still attached to the calculator and that his/her study list ...
Program Scheme - Manipal University Jaipur
... 2. Verma and Srivastava, Crystallography for Solid State Physics, New age publication, 1991 3. Kittle, Solid State Physics, Wiley, 8th ed., (2004) 4. Wahab M.A., Solid State Physics, Alpha Science international Ltd, 1 st ed, (2011) 7. Chaikin and Lubensky, Principals of Condensed Mater Physics, Camb ...
... 2. Verma and Srivastava, Crystallography for Solid State Physics, New age publication, 1991 3. Kittle, Solid State Physics, Wiley, 8th ed., (2004) 4. Wahab M.A., Solid State Physics, Alpha Science international Ltd, 1 st ed, (2011) 7. Chaikin and Lubensky, Principals of Condensed Mater Physics, Camb ...
χSR - MENU 2013
... predicted (long ago) to exist as a chiral partner of the NambuGoldstone π meson, corresponding to the dynamical breaking of chiral symmetry (conserving in the massless limit of QCD), with mass mσ ≈ 2mq (mq is the constituent quark mass). ...
... predicted (long ago) to exist as a chiral partner of the NambuGoldstone π meson, corresponding to the dynamical breaking of chiral symmetry (conserving in the massless limit of QCD), with mass mσ ≈ 2mq (mq is the constituent quark mass). ...
Life of The Cosmos By
... pressure in the center is strong enough that the nuclear reactions ignite. As a result, the number of atoms necessary to make a star turns out to grow as the gravitational constant decreases. Stars are so huge exactly because the gravitational constant is so tiny. It is fortunate for us that stars a ...
... pressure in the center is strong enough that the nuclear reactions ignite. As a result, the number of atoms necessary to make a star turns out to grow as the gravitational constant decreases. Stars are so huge exactly because the gravitational constant is so tiny. It is fortunate for us that stars a ...
Forces Webquest Focus Questions
... If there was no friction between a match and the match box surface, would the match light? Explain your answer. ...
... If there was no friction between a match and the match box surface, would the match light? Explain your answer. ...
Static Electricity
... of 3.4 grams. Checking the periodic table, we find that the atomic number of copper is 29 and its atomic mass is 63.546. This means that 6.022 x 1023 atoms of copper (= 1 mole = Avogadro’s number) has a mass of 63.546 grams, and each individual atom contains 29 protons, 29 electrons, and on average, ...
... of 3.4 grams. Checking the periodic table, we find that the atomic number of copper is 29 and its atomic mass is 63.546. This means that 6.022 x 1023 atoms of copper (= 1 mole = Avogadro’s number) has a mass of 63.546 grams, and each individual atom contains 29 protons, 29 electrons, and on average, ...
Fundamental Particles, Fundamental Questions
... radioactive decays would not conserve energy or momentum. ...
... radioactive decays would not conserve energy or momentum. ...
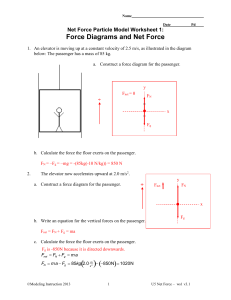
Net Force, Mass and Acceleration activity
... We have already studied objects at rest and learned that they have a total – or ‘net’ – force of zero; that any forces in a particular direction are canceled out by one or more forces in the opposite direction. Purpose: This activity will help you investigate the effect of unbalanced forces, net for ...
... We have already studied objects at rest and learned that they have a total – or ‘net’ – force of zero; that any forces in a particular direction are canceled out by one or more forces in the opposite direction. Purpose: This activity will help you investigate the effect of unbalanced forces, net for ...
Chapter 31
... the same atomic number. In other words, the number of protons an atom has defines what kind of element it is. The total number of neutrons and protons in an atom is called the mass number (A) of that element. The symbol ZA X is used to show both the atomic number and the mass number of an X atom, wh ...
... the same atomic number. In other words, the number of protons an atom has defines what kind of element it is. The total number of neutrons and protons in an atom is called the mass number (A) of that element. The symbol ZA X is used to show both the atomic number and the mass number of an X atom, wh ...
Conception of Generations
... mentioned above qualitatively. In 1933 immediately after this discovery Heisenberg and Ivanenko independently proposed that nuclei are composed of protons and neutrons. This new nuclear model and Pauli’s “neutrino” were merged by Fermi1) to account for the beta-decay in 1934. He introduced a field-th ...
... mentioned above qualitatively. In 1933 immediately after this discovery Heisenberg and Ivanenko independently proposed that nuclei are composed of protons and neutrons. This new nuclear model and Pauli’s “neutrino” were merged by Fermi1) to account for the beta-decay in 1934. He introduced a field-th ...
Centripetal and Gravitational Forces
... Centrifugal force ("fictitious" force) represents the effects of inertia that arise in connection with rotation It is experienced as an outward force away from the center of rotation. ...
... Centrifugal force ("fictitious" force) represents the effects of inertia that arise in connection with rotation It is experienced as an outward force away from the center of rotation. ...
Nuclear force

The nuclear force (or nucleon–nucleon interaction or residual strong force) is the force between protons and neutrons, subatomic particles that are collectively called nucleons. The nuclear force is responsible for binding protons and neutrons into atomic nuclei. Neutrons and protons are affected by the nuclear force almost identically. Since protons have charge +1 e, they experience a Coulomb repulsion that tends to push them apart, but at short range the nuclear force is sufficiently attractive as to overcome the electromagnetic repulsive force. The mass of a nucleus is less than the sum total of the individual masses of the protons and neutrons which form it. The difference in mass between bound and unbound nucleons is known as the mass defect. Energy is released when nuclei break apart, and it is this energy that used in nuclear power and nuclear weapons.The nuclear force is powerfully attractive between nucleons at distances of about 1 femtometer (fm, or 1.0 × 10−15 metres) between their centers, but rapidly decreases to insignificance at distances beyond about 2.5 fm. At distances less than 0.7 fm, the nuclear force becomes repulsive. This repulsive component is responsible for the physical size of nuclei, since the nucleons can come no closer than the force allows. By comparison, the size of an atom, measured in angstroms (Å, or 1.0 × 10−10 m), is five orders of magnitude larger. The nuclear force is not simple, however, since it depends on the nucleon spins, has a tensor component, and may depend on the relative momentum of the nucleons.A quantitative description of the nuclear force relies on partially empirical equations that model the internucleon potential energies, or potentials. (Generally, forces within a system of particles can be more simply modeled by describing the system's potential energy; the negative gradient of a potential is equal to the vector force.) The constants for the equations are phenomenological, that is, determined by fitting the equations to experimental data. The internucleon potentials attempt to describe the properties of nucleon–nucleon interaction. Once determined, any given potential can be used in, e.g., the Schrödinger equation to determine the quantum mechanical properties of the nucleon system.The discovery of the neutron in 1932 revealed that atomic nuclei were made of protons and neutrons, held together by an attractive force. By 1935 the nuclear force was conceived to be transmitted by particles called mesons. This theoretical development included a description of the Yukawa potential, an early example of a nuclear potential. Mesons, predicted by theory, were discovered experimentally in 1947. By the 1970s, the quark model had been developed, which showed that the mesons and nucleons were composed of quarks and gluons. By this new model, the nuclear force, resulting from the exchange of mesons between neighboring nucleons, is a residual effect of the strong force.