Summary of Class 4 8.02 Tuesday 2/8/05 / Wednesday 2/9/05 Topics
... the TEAL visualizations and how to use them, in Experiment 1. We then turn to the concept of electric potential. Just as electric fields are analogous to gravitational fields, electric potential is analogous to gravitational potential. We introduce from the point of view of calculating the electric ...
... the TEAL visualizations and how to use them, in Experiment 1. We then turn to the concept of electric potential. Just as electric fields are analogous to gravitational fields, electric potential is analogous to gravitational potential. We introduce from the point of view of calculating the electric ...
TYPES OF ENERGY
... (Radiant) Energy • Plants use light (radiant) energy to make chemical energy. [remember Photosynthesis] • The chemical energy in food is then changed into another kind of chemical energy that your body can use. [remember cellular respiration] • Your body then uses that energy to give you mechanical ...
... (Radiant) Energy • Plants use light (radiant) energy to make chemical energy. [remember Photosynthesis] • The chemical energy in food is then changed into another kind of chemical energy that your body can use. [remember cellular respiration] • Your body then uses that energy to give you mechanical ...
PH504lec0809-6
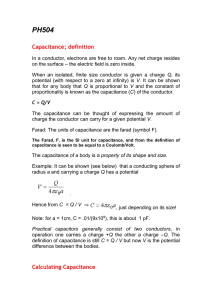
... When an isolated, finite size conductor is given a charge Q, its potential (with respect to a zero at infinity) is V. It can be shown that for any body that Q is proportional to V and the constant of proportionality is known as the capacitance (C) of the conductor. C = Q/V The capacitance can be tho ...
... When an isolated, finite size conductor is given a charge Q, its potential (with respect to a zero at infinity) is V. It can be shown that for any body that Q is proportional to V and the constant of proportionality is known as the capacitance (C) of the conductor. C = Q/V The capacitance can be tho ...
Conservation of energy∗
... electric and such other changes called processes. For example, a body may not involve motion as a whole, but atoms/molecules constituting the body may be undergoing motion all the time. For example, work for gas compression does not involve locomotion of the gas mass. It brings about change in inter ...
... electric and such other changes called processes. For example, a body may not involve motion as a whole, but atoms/molecules constituting the body may be undergoing motion all the time. For example, work for gas compression does not involve locomotion of the gas mass. It brings about change in inter ...
1. Principles of Thermodynamics
... in many cases like the prototypical one-component gas two is enough to determine the equilibrium state, in which the rest are then functions of these parameters, state functions. State variables are either extensive or intensive, the former being proportional to the number of particles (the volume V ...
... in many cases like the prototypical one-component gas two is enough to determine the equilibrium state, in which the rest are then functions of these parameters, state functions. State variables are either extensive or intensive, the former being proportional to the number of particles (the volume V ...
Energy changes forms.
... When energy changes forms, the total amount of energy is conserved. However, the amount of useful energy is almost always less than the total amount of energy. For example, consider the energy used by an electric fan. The amount of electrical energy used is greater than the kinetic energy of the mov ...
... When energy changes forms, the total amount of energy is conserved. However, the amount of useful energy is almost always less than the total amount of energy. For example, consider the energy used by an electric fan. The amount of electrical energy used is greater than the kinetic energy of the mov ...
Matter and Energy
... 2. There is no relationship between matter and energy. 3. If energy is conserved, why are we running out of it? 4. Energy can be changed completely from one form to another (no energy losses). 5. Things “use up” energy. 6. Energy is confined to some particular origin, such as what we get from food o ...
... 2. There is no relationship between matter and energy. 3. If energy is conserved, why are we running out of it? 4. Energy can be changed completely from one form to another (no energy losses). 5. Things “use up” energy. 6. Energy is confined to some particular origin, such as what we get from food o ...
Chapter 16
... It moves from a point of higher potential to a point of lower potential Its electrical potential energy decreases Its kinetic energy increases Obeys conservation of energy relationship: ...
... It moves from a point of higher potential to a point of lower potential Its electrical potential energy decreases Its kinetic energy increases Obeys conservation of energy relationship: ...
Chapter 6 Energy PPT
... • Kinetic energy also can be transformed into potential energy. • Suppose you throw a ball straight up into the air. • The muscles in your body cause the ball to move upward when it leaves your hand. • Because it is moving, the ball has kinetic energy. ...
... • Kinetic energy also can be transformed into potential energy. • Suppose you throw a ball straight up into the air. • The muscles in your body cause the ball to move upward when it leaves your hand. • Because it is moving, the ball has kinetic energy. ...
B - Purdue Physics
... Electric Potential Energy of a Charge in Electric Field • Coulomb force is conservative => Work done by the Coulomb force is path independent. ...
... Electric Potential Energy of a Charge in Electric Field • Coulomb force is conservative => Work done by the Coulomb force is path independent. ...
ExamView - Quiz 3--Heat and Thermo PRACTICE.tst
... 28. The requirement that a heat engine must give up some energy at a lower temperature in order to do work corresponds to which law of thermodynamics? a. first b. second c. third d. No law of thermodynamics applies. 29. A heat engine has taken in energy as heat and used a portion of it to do work. W ...
... 28. The requirement that a heat engine must give up some energy at a lower temperature in order to do work corresponds to which law of thermodynamics? a. first b. second c. third d. No law of thermodynamics applies. 29. A heat engine has taken in energy as heat and used a portion of it to do work. W ...
5.2 Energy in Mechanical and Fluid Systems II
... when other types of forces act on it. As with gravity, these forces have magnitudes that depend on the object’s position. For example, when you stretch a rubber band it exerts a restoring force that increases in magnitude as you increase the distance stretched. When you release the rubber band, it r ...
... when other types of forces act on it. As with gravity, these forces have magnitudes that depend on the object’s position. For example, when you stretch a rubber band it exerts a restoring force that increases in magnitude as you increase the distance stretched. When you release the rubber band, it r ...