Complexometric Reactions and Titrations
... This can be simply answered by looking at the thermodynamics of the process. We know from simple thermodynamics that spontaneous processes are favored if an increase in entropy results. Now look at the dissociation of the chelate and the complex mentioned above, dissociation of the chelate will giv ...
... This can be simply answered by looking at the thermodynamics of the process. We know from simple thermodynamics that spontaneous processes are favored if an increase in entropy results. Now look at the dissociation of the chelate and the complex mentioned above, dissociation of the chelate will giv ...
InorgCh425
... b) p back-bonding increases the ligand strength c) These are the same p bonds that Y must use as it approaches to bond ...
... b) p back-bonding increases the ligand strength c) These are the same p bonds that Y must use as it approaches to bond ...
ncur_powerpoint Courtney
... Typical Metal Complex: [Ni3(tris-CB-Cyclens)(OAc)3](PF6)3 ∙ 6H2O Elemental Analysis Calculated as Ni3C48H87N12O6P3F18 ∙ 6 H2O: Calc: C 35.00, H 6.06, N 10.20; Found: C 34.66, H 5.61, N 10.20 ...
... Typical Metal Complex: [Ni3(tris-CB-Cyclens)(OAc)3](PF6)3 ∙ 6H2O Elemental Analysis Calculated as Ni3C48H87N12O6P3F18 ∙ 6 H2O: Calc: C 35.00, H 6.06, N 10.20; Found: C 34.66, H 5.61, N 10.20 ...
Structural Preferences of N-Substituted Monosaccharide Derivatives
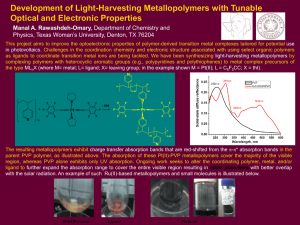
... Physics, Texas Woman’s University, Denton, TX 76204 This project aims to improve the optoelectronic properties of polymer-derived transition metal complexes tailored for potential use in photovoltaics. Challenges in the coordination chemistry and electronic structure associated with using select org ...
... Physics, Texas Woman’s University, Denton, TX 76204 This project aims to improve the optoelectronic properties of polymer-derived transition metal complexes tailored for potential use in photovoltaics. Challenges in the coordination chemistry and electronic structure associated with using select org ...
Document
... • Formation of chelate ring ⇒ reaction proceeds in forward direction & the product is stable. This stability is purely kinetic in nature. This is known as chelate effect. • ΔG = ΔH - TΔS ...
... • Formation of chelate ring ⇒ reaction proceeds in forward direction & the product is stable. This stability is purely kinetic in nature. This is known as chelate effect. • ΔG = ΔH - TΔS ...
Studying Transition Metal Complexes
... The equation describing this would be: Co+3 + 6NH3 Co(NH3)6+3. Metallic elements tend to form 2, 4, or 6 bonds with ligands. The number of bonds formed is called the coordination number. Iron in oxyhemoglobin, cobalt in Vitamin B12, and magnesium in chlorophyll all have coordination number 6. Copp ...
... The equation describing this would be: Co+3 + 6NH3 Co(NH3)6+3. Metallic elements tend to form 2, 4, or 6 bonds with ligands. The number of bonds formed is called the coordination number. Iron in oxyhemoglobin, cobalt in Vitamin B12, and magnesium in chlorophyll all have coordination number 6. Copp ...
Thermodynamics and kinetics
... • The best buffer is when [HA]=[A-] largest buffer range for the conditions pH = pKa - log1 • For a buffer the range is determined by [HA]/[A-] [HA]/[A-] from 0.1 to 10 Buffer pH range = pKa ± 1 Higher buffer concentration increase durability ...
... • The best buffer is when [HA]=[A-] largest buffer range for the conditions pH = pKa - log1 • For a buffer the range is determined by [HA]/[A-] [HA]/[A-] from 0.1 to 10 Buffer pH range = pKa ± 1 Higher buffer concentration increase durability ...
Supramolecular photochemistry
... and longer the excited state lifetime. It may also happen, however, that the cage ligand does not allow the metal to attain an appropriate octahedral coordination geometry and/or suitable Ru-N bond distances. Molecular models show that the cryptand of 6 is suitable for the larger, not symmetry-deman ...
... and longer the excited state lifetime. It may also happen, however, that the cage ligand does not allow the metal to attain an appropriate octahedral coordination geometry and/or suitable Ru-N bond distances. Molecular models show that the cryptand of 6 is suitable for the larger, not symmetry-deman ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... CH 3809 - COORDINATION CHEMISTRY Date : 05/11/2013 Time : 9:00 - 12:00 ...
... CH 3809 - COORDINATION CHEMISTRY Date : 05/11/2013 Time : 9:00 - 12:00 ...
Homework 5 Insert space or answer ques
... Be SURE to identify which d orbitals are located where!! 5.) What is the distinction between a weak field and a strong field ligand? What does it mean for electrons filling the d orbitals? Give an example of a weak field ligand. Give an example of a strong field ligand ...
... Be SURE to identify which d orbitals are located where!! 5.) What is the distinction between a weak field and a strong field ligand? What does it mean for electrons filling the d orbitals? Give an example of a weak field ligand. Give an example of a strong field ligand ...
axial - TAMU Chemistry
... first, then when more ligand is added, [ML2] rises sharply & [ML] drops. With more added L, [ML2] drops and [ML3] rises etc., etc., Since the ligand addition to form a new complex is always reversible, an MLn progresses with greater n values, there are more ligands to fall back off and fewer places ...
... first, then when more ligand is added, [ML2] rises sharply & [ML] drops. With more added L, [ML2] drops and [ML3] rises etc., etc., Since the ligand addition to form a new complex is always reversible, an MLn progresses with greater n values, there are more ligands to fall back off and fewer places ...
Chapter 24: Transition Metals Coordination Compounds Part 1
... So you could easily form the salt [Ag(NH3)2]Cl. Note how the Cl- anion is outside the brackets. The coordination number of a coordination compound is simply the number of ligand atoms which are directly covalently attached to the transition metal atom or ion. So the coordination number of [Ag(NH3)2] ...
... So you could easily form the salt [Ag(NH3)2]Cl. Note how the Cl- anion is outside the brackets. The coordination number of a coordination compound is simply the number of ligand atoms which are directly covalently attached to the transition metal atom or ion. So the coordination number of [Ag(NH3)2] ...
Lanthanum has only one important oxidation state in aqueous
... • Whole ion written in brackets – may be cationic or anionic ...
... • Whole ion written in brackets – may be cationic or anionic ...
TRANSITION METAL CHEMISTRY –PART 3 –class notes
... 4. Which of the following octahedral complexes should have the largest crystal field splitting energy? A) [Cr(NH3]3+ B) [CrF6]3- C) [Cr(H2O)]3+ D) [Cr(CN)6]3- E) [CrCl6]35. CN- is a strong field ligand, whereas Cl- is usually a weak field ligand. Which of the following octahedral complexes has no un ...
... 4. Which of the following octahedral complexes should have the largest crystal field splitting energy? A) [Cr(NH3]3+ B) [CrF6]3- C) [Cr(H2O)]3+ D) [Cr(CN)6]3- E) [CrCl6]35. CN- is a strong field ligand, whereas Cl- is usually a weak field ligand. Which of the following octahedral complexes has no un ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... geometry, and compute the field strength of the axial and equatorial ligands. 24a. The epr spectrum of a high-spin manganese(II) complex doped into a diamagnetic host consists of 30 lines (five sets of six lines each). ...
... geometry, and compute the field strength of the axial and equatorial ligands. 24a. The epr spectrum of a high-spin manganese(II) complex doped into a diamagnetic host consists of 30 lines (five sets of six lines each). ...
SC-Database
... dependence may be calculated from built-in routines (Davies and van’t Hoff equations): ...
... dependence may be calculated from built-in routines (Davies and van’t Hoff equations): ...
Nomenclature for d-block complexes Writing chemical names
... (also known as, “how to name compounds and write formulae”) ...
... (also known as, “how to name compounds and write formulae”) ...