2 ATP - HONORS BIOLOGY
... Occurs in the Matrix of the Mitochondria Everything in the Krebs Cycle happens twice because there are two pyruvates from glycolysis. 1) Pyruvate enters the mitochondria and immediately loses a CO2 and makes a NADH forming acetic acid which binds to an enzyme called Co-enzyme A. CO2 Acetic Acid + Co ...
... Occurs in the Matrix of the Mitochondria Everything in the Krebs Cycle happens twice because there are two pyruvates from glycolysis. 1) Pyruvate enters the mitochondria and immediately loses a CO2 and makes a NADH forming acetic acid which binds to an enzyme called Co-enzyme A. CO2 Acetic Acid + Co ...
200 µmol /L is far too low a concentration of ammonium to affect
... reaction is readily reversible, and the direction of reaction (towards deamination of glutamate or glutamate formation) depends on the relative concentrations of the various substrates. As the concentration of ammonium rises, so the reaction proceeds in the direction of formation of glutamate from k ...
... reaction is readily reversible, and the direction of reaction (towards deamination of glutamate or glutamate formation) depends on the relative concentrations of the various substrates. As the concentration of ammonium rises, so the reaction proceeds in the direction of formation of glutamate from k ...
Respiration
... By means of a flow diagram, briefly outline the three main stages of aerobic respiration with a carbohydrate substrate. Annotate your diagram to show the essential features of each of the three main stages.
[7 marks]
(94 II 2)
(a) De ...
... By means of a flow diagram, briefly outline the three main stages of aerobic respiration with a carbohydrate substrate. Annotate your diagram to show the essential features of each of the three main stages.
Amino Acids - Rose
... equal magnitudes, the molecule has zero net charge. This zero net charge state is known as the zwitterionic state (a zwitterion is capable of both accepting and donating protons), and is characteristic of free amino acids with non-ionizable sidechains near pH 7.0. As the pH increases further, the ch ...
... equal magnitudes, the molecule has zero net charge. This zero net charge state is known as the zwitterionic state (a zwitterion is capable of both accepting and donating protons), and is characteristic of free amino acids with non-ionizable sidechains near pH 7.0. As the pH increases further, the ch ...
Document
... • To balance a chemical equation • begin with atoms that appear only in one compound on the left and one on the right; in this case, begin with carbon (C) which occurs in C3H8 and CO2 C3 H8 (g) + O2 (g) ...
... • To balance a chemical equation • begin with atoms that appear only in one compound on the left and one on the right; in this case, begin with carbon (C) which occurs in C3H8 and CO2 C3 H8 (g) + O2 (g) ...
- DigitalCommons@USU
... symmetry of MPc 共in solution兲 is D 4h . The molecular structure of MPc is illustrated in Fig. 1 wherein the central metal 共M兲 is coordinated to four N atoms. Full geometry optimization of each MPc under D 2h symmetry constraints leads to structures that are very close to the expected D 4h symmetry: ...
... symmetry of MPc 共in solution兲 is D 4h . The molecular structure of MPc is illustrated in Fig. 1 wherein the central metal 共M兲 is coordinated to four N atoms. Full geometry optimization of each MPc under D 2h symmetry constraints leads to structures that are very close to the expected D 4h symmetry: ...
NCERT Solution - Mywayteaching
... Lattice energy is directly proportional to the charge carried by an ion. When a metal combines with oxygen, the lattice energy of the oxide involving O2− ion is much more than the oxide involving O− ion. Hence, the oxide having O2− ions are more stable than oxides having O−. Hence, we can say that f ...
... Lattice energy is directly proportional to the charge carried by an ion. When a metal combines with oxygen, the lattice energy of the oxide involving O2− ion is much more than the oxide involving O− ion. Hence, the oxide having O2− ions are more stable than oxides having O−. Hence, we can say that f ...
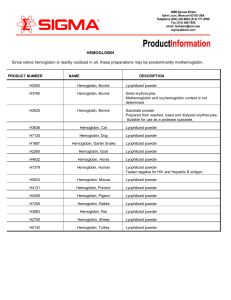
HEMOGLOBIN Since native hemoglobin is readily oxidized in air
... Hemoglobin is the major component of red blood cells, and is responsible for their red color. Its normal concentration in erythrocytes is 34%. Hemoglobin is the most important respiratory protein of vertebrates by virtue of its ability to transport oxygen from the lungs to body tissues, and to facil ...
... Hemoglobin is the major component of red blood cells, and is responsible for their red color. Its normal concentration in erythrocytes is 34%. Hemoglobin is the most important respiratory protein of vertebrates by virtue of its ability to transport oxygen from the lungs to body tissues, and to facil ...
Enter Legible BANNER ID: B 0 0 __ __ __ __ __ __ DO NOT WRITE
... Noncyclic photophosphorylation ...
... Noncyclic photophosphorylation ...
Analyzing Chemical Equations ANSWER KEY
... present in the reactants? present in the products? C–6 C–6 H – 12 H – 12 O – 18 O – 18 Is this a balanced equation? Explain. YES! The number of atoms for each element is equal in the reactants and the products. 5. Plant cells use water, carbon dioxide, and energy from the sun to produce glucose and ...
... present in the reactants? present in the products? C–6 C–6 H – 12 H – 12 O – 18 O – 18 Is this a balanced equation? Explain. YES! The number of atoms for each element is equal in the reactants and the products. 5. Plant cells use water, carbon dioxide, and energy from the sun to produce glucose and ...
Determination of the Binding Site-Size of the Protein
... crude cell extracts, it is thought that cell extracts contain many other DNA-binding proteins (and their regulatory proteins), and some small molecules (such as ATP and DNA metabolites), that are also able to interact with the DNA and the target protein; these molecules may cause some unpredictable ...
... crude cell extracts, it is thought that cell extracts contain many other DNA-binding proteins (and their regulatory proteins), and some small molecules (such as ATP and DNA metabolites), that are also able to interact with the DNA and the target protein; these molecules may cause some unpredictable ...
... Amino acid 2. Only the protonated form of histidine can participate acid in protein in electrostatic interactions, therefore any effects Energy HA A A HA on the deprotonated form can be ignored. 3. The negative charge on the aspartic acid residue will stabilize the + charge on the histidine side cha ...
Amino Acid Metabolism (day-2)
... all other needed N metabolites • In these organisms, glutamate is the source of N, via transamination (aminotransferase) reactions of α-keto acid analogue of the amino acid • Mammals can make only 10 of the 20 amino acids • The others are classed as "essential" amino acids and must be obtained in th ...
... all other needed N metabolites • In these organisms, glutamate is the source of N, via transamination (aminotransferase) reactions of α-keto acid analogue of the amino acid • Mammals can make only 10 of the 20 amino acids • The others are classed as "essential" amino acids and must be obtained in th ...
DOC
... food conversion (maximise protein deposition in the animal) and to maximise growth performance under culture conditions. Understanding the physiological basis of observed growth in terms of anabolic and catabolic processes will then enable informed decisions to be made on the modification of diets a ...
... food conversion (maximise protein deposition in the animal) and to maximise growth performance under culture conditions. Understanding the physiological basis of observed growth in terms of anabolic and catabolic processes will then enable informed decisions to be made on the modification of diets a ...
Mineral Tutorial Definition of a mineral: A mineral substance is a
... Definition of a mineral: A mineral substance is a naturally occurring solid that has been formed by geological processes, either on earth or in extraterrestrial bodies (Nickel 1995a). A mineral has a well-defined chemical composition and crystallographic properties, and which merits a unique name an ...
... Definition of a mineral: A mineral substance is a naturally occurring solid that has been formed by geological processes, either on earth or in extraterrestrial bodies (Nickel 1995a). A mineral has a well-defined chemical composition and crystallographic properties, and which merits a unique name an ...
www.xtremepapers.net
... as a flux for soldering and tinning, as a corrosion inhibitor in cooling towers and in the manufacture of rayon. (a) Draw a fully labelled diagram to show how you could use a standard hydrogen electrode to measure the standard electrode potential, E o, of zinc. ...
... as a flux for soldering and tinning, as a corrosion inhibitor in cooling towers and in the manufacture of rayon. (a) Draw a fully labelled diagram to show how you could use a standard hydrogen electrode to measure the standard electrode potential, E o, of zinc. ...
Chapter 9 Review, pages 628–633
... Fe(s) → Fe2+(aq) The elements have been balanced. Use electrons to balance the charge. Therefore, the half-reaction equations for the redox reaction are Oxidation: Fe(s) → Fe2+(aq) + 2 e− Reduction: 2 HNO2(aq) + 4 H+(aq) + 4 e− → N2O(g) + 3 H2O(l) (c) Separate O2(g) + 2 H2O(l) + Co(s) → Co2+(aq) + 4 ...
... Fe(s) → Fe2+(aq) The elements have been balanced. Use electrons to balance the charge. Therefore, the half-reaction equations for the redox reaction are Oxidation: Fe(s) → Fe2+(aq) + 2 e− Reduction: 2 HNO2(aq) + 4 H+(aq) + 4 e− → N2O(g) + 3 H2O(l) (c) Separate O2(g) + 2 H2O(l) + Co(s) → Co2+(aq) + 4 ...
study guide
... High-Energy Electrons The energy in light raises some of the electrons in chlorophyll to higher energy levels. These high-energy electrons are used in photosynthesis. Electron carriers are used to transport the electrons from chlorophyll to other molecules during photosynthesis. NADP+ is a compound ...
... High-Energy Electrons The energy in light raises some of the electrons in chlorophyll to higher energy levels. These high-energy electrons are used in photosynthesis. Electron carriers are used to transport the electrons from chlorophyll to other molecules during photosynthesis. NADP+ is a compound ...
Contents - motensar memorial arts and science college sirsi
... complexes containing primary and secondary amines were also found to be clinically active. The N-H bonds in these complexes appear to play a crucial role in their mechanism of action. Other complexes of Pt (II) with biologically active N-donor heterocyclic ligands have shown cytotoxicity. The precis ...
... complexes containing primary and secondary amines were also found to be clinically active. The N-H bonds in these complexes appear to play a crucial role in their mechanism of action. Other complexes of Pt (II) with biologically active N-donor heterocyclic ligands have shown cytotoxicity. The precis ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.