Is gold always chemically passive? Study and comparison of the
... topology, dynamics and three-dimensional structure of molecules in a solution and in the solid state. It is a very selective technique, distinguishing among many atoms within a molecule or collection of molecules, the same type of atoms that differ only in terms of their local chemical environment: ...
... topology, dynamics and three-dimensional structure of molecules in a solution and in the solid state. It is a very selective technique, distinguishing among many atoms within a molecule or collection of molecules, the same type of atoms that differ only in terms of their local chemical environment: ...
A modular approach to neutral P,N
... cationic palladium species [2a-PdCl]BF4, which is dimeric in the solid state [11]. Similarly, Pd(allyl) complexes of ligands 2a, 5 and 7 were obtained from mixtures of the respective ligand, [Pd(allyl)Cl]2 and AgBF4 or AgOTf as the chloride scavenger in DCM (Figure 5B). We were able to obtain single ...
... cationic palladium species [2a-PdCl]BF4, which is dimeric in the solid state [11]. Similarly, Pd(allyl) complexes of ligands 2a, 5 and 7 were obtained from mixtures of the respective ligand, [Pd(allyl)Cl]2 and AgBF4 or AgOTf as the chloride scavenger in DCM (Figure 5B). We were able to obtain single ...
ligand design - UZH - Department of Chemistry
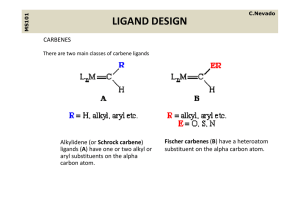
... containing pi acceptor ligands These are electrophilic at the alpha carbon count as neutral two containing pi‐acceptor ligands. These are electrophilic at the alpha‐carbon, count as neutral two‐ electron donor ligands, their ligation being similar to CO. We can draw a resonance structure as follows ...
... containing pi acceptor ligands These are electrophilic at the alpha carbon count as neutral two containing pi‐acceptor ligands. These are electrophilic at the alpha‐carbon, count as neutral two‐ electron donor ligands, their ligation being similar to CO. We can draw a resonance structure as follows ...
Lecture 10 -Further Consequences of d
... iv. The crystal field stabilization energy (CFSE). It is possible to calculate CFSE's CFSE s in any geometry in terms of the octahedral splitting energy o; we've seen how to do it for three common geometries: octahedral, tetrahedral, and square planar. Clearly, the CFSE is important, and it depends ...
... iv. The crystal field stabilization energy (CFSE). It is possible to calculate CFSE's CFSE s in any geometry in terms of the octahedral splitting energy o; we've seen how to do it for three common geometries: octahedral, tetrahedral, and square planar. Clearly, the CFSE is important, and it depends ...
Molybdenum-Pterin Chemistry. 3. Use of X
... supported by spectroscopic and microanalytical data. X-ray Photoelectron Spectroscopy (XPS). This method allows the determination of the oxidation state of a metal ion provided the compound is reasonably stable and standard compounds are available. The energy scale of the spectra can be shifted due ...
... supported by spectroscopic and microanalytical data. X-ray Photoelectron Spectroscopy (XPS). This method allows the determination of the oxidation state of a metal ion provided the compound is reasonably stable and standard compounds are available. The energy scale of the spectra can be shifted due ...
Chromium Chemistry
... It is the longest-known example of a family of compounds that have the general formula Cr2(O2CR)4L2, which have the following structure: ...
... It is the longest-known example of a family of compounds that have the general formula Cr2(O2CR)4L2, which have the following structure: ...
Photochemistry of tetrasulfido complexes of molybdenum (VI
... ligands to the metal.' However, a t these high formal oxidation states of the metal, the bonding in these complexes must have large covalent contributions. I t follows that some of the MO's are strongly delocalized between metal and ligands. L M C T transitions may then not be associated with the tr ...
... ligands to the metal.' However, a t these high formal oxidation states of the metal, the bonding in these complexes must have large covalent contributions. I t follows that some of the MO's are strongly delocalized between metal and ligands. L M C T transitions may then not be associated with the tr ...
THE ELECTROCHEMISTRY OF STRAPPED AND CAPPED PORPHYRIN
... may be explained by two plausible mechanisms: an EE reaction to form radical-cation and dlcationic species or an ECE mechanism in which the chemical process (e.g. a cleavage of the strap and/or loss of the conjugation in the porphyrin ring) is very fast relative to the scan rate [13]. In this case t ...
... may be explained by two plausible mechanisms: an EE reaction to form radical-cation and dlcationic species or an ECE mechanism in which the chemical process (e.g. a cleavage of the strap and/or loss of the conjugation in the porphyrin ring) is very fast relative to the scan rate [13]. In this case t ...
The Influence of a New-Synthesized Complex Compounds of
... of us are aware that "organic perchlorates are self-contained explosives15 However, many overlook the fact that a perchlorate salt of a cation, such as a complex ion that contains an organic group or other oxidizable atoms, is also an explosive (although the conditions required to initiate an explos ...
... of us are aware that "organic perchlorates are self-contained explosives15 However, many overlook the fact that a perchlorate salt of a cation, such as a complex ion that contains an organic group or other oxidizable atoms, is also an explosive (although the conditions required to initiate an explos ...
UV-Visible Spectra of Aquavanadium Complexes
... Vanadium plays a number of roles in the biological systems including its presence in two enzymes, vanadium dependent haloperoxidases and nitrogenase. In the human organism, it elicits a number of physiological responses, including the inhibition of phosphate-metabolizing enzymes, such as phosphatase ...
... Vanadium plays a number of roles in the biological systems including its presence in two enzymes, vanadium dependent haloperoxidases and nitrogenase. In the human organism, it elicits a number of physiological responses, including the inhibition of phosphate-metabolizing enzymes, such as phosphatase ...
Received 02-11-2001
... Results and discussion Table 1 summarizes the carbon, hydrogen and nitrogen elemental analysis of the isolated complexes. The results obtained indicate that all of the isolated complexes are formed from the reaction of the metal salt with drug in 1:2 molar ratio. All of the complexes reported herein ...
... Results and discussion Table 1 summarizes the carbon, hydrogen and nitrogen elemental analysis of the isolated complexes. The results obtained indicate that all of the isolated complexes are formed from the reaction of the metal salt with drug in 1:2 molar ratio. All of the complexes reported herein ...
Substitution reactions
... σ-donor ligands Î Ligands capable of contributing more electron density to the shared orbital between itself and the trans ligand, thereby weakening the bond to the leaving group Î trans influence π-acceptor ligands Î drain away electrons in A or Ia substitution reactions Î TS characterised by high ...
... σ-donor ligands Î Ligands capable of contributing more electron density to the shared orbital between itself and the trans ligand, thereby weakening the bond to the leaving group Î trans influence π-acceptor ligands Î drain away electrons in A or Ia substitution reactions Î TS characterised by high ...
Welcome to CHMC31 course, a course that brings to you... world of transition elements. Below you will find a more... Intermediate Inorganic Chemistry (CHMC31Y3)
... chemistry. General topics will include: overview of transition metal properties (their position in the Periodic Table of Elements, relationships to the main group elements, etc.) main classes of compounds, coordination compounds (structure and bonding, general reactivity, magnetic properties), spect ...
... chemistry. General topics will include: overview of transition metal properties (their position in the Periodic Table of Elements, relationships to the main group elements, etc.) main classes of compounds, coordination compounds (structure and bonding, general reactivity, magnetic properties), spect ...
Intermediate Inorganic Chemistry (CHMC39Y)
... chemistry. General topics will include: overview of transition metal properties (their position in the Periodic Table of Elements, relationships to the main group elements, etc.) main classes of compounds, coordination compounds (structure and bonding, general reactivity, magnetic properties), spect ...
... chemistry. General topics will include: overview of transition metal properties (their position in the Periodic Table of Elements, relationships to the main group elements, etc.) main classes of compounds, coordination compounds (structure and bonding, general reactivity, magnetic properties), spect ...
Carbonylation catalyst system
... group having formula (I) as indicated above, may coor dinate to a plurality of Group VIII metal atoms, and accordingly can be used at a lower ligand to metal ratio. In a preferred embodiment, the catalyst system ac cording to the invention further comprises a protonic acid. The function of the proto ...
... group having formula (I) as indicated above, may coor dinate to a plurality of Group VIII metal atoms, and accordingly can be used at a lower ligand to metal ratio. In a preferred embodiment, the catalyst system ac cording to the invention further comprises a protonic acid. The function of the proto ...
Inglés - SciELO Argentina
... Nitroprusside, [Fe(CN)5NO]2- (NP), first prepared in the middle of 19th century, has a special place among iron-nitrosyl complexes, mainly after the discovery, in 1929, of its effective hypotensive properties. NP is routinely used in clinical studies as an NO-donor drug, although the mechanistic det ...
... Nitroprusside, [Fe(CN)5NO]2- (NP), first prepared in the middle of 19th century, has a special place among iron-nitrosyl complexes, mainly after the discovery, in 1929, of its effective hypotensive properties. NP is routinely used in clinical studies as an NO-donor drug, although the mechanistic det ...
SYNTHESIS AND ANTIMICROBIAL STUDIES OF IRON (III
... EDTA. The purpose of this application of complexation is to improve drug stability by inhibiting reactions (usually oxidations) that are catalyzed by metal ions, the complexed form of the metal ion being catalytically inactive. Citric acid (in the form of citrate anion) also is used for this purpose ...
... EDTA. The purpose of this application of complexation is to improve drug stability by inhibiting reactions (usually oxidations) that are catalyzed by metal ions, the complexed form of the metal ion being catalytically inactive. Citric acid (in the form of citrate anion) also is used for this purpose ...
click - Chemsheets
... • For some ions, a ligand is added to intensify the colour. • The strength of absorption of solutions of known concentration is measured and a graph produced. ...
... • For some ions, a ligand is added to intensify the colour. • The strength of absorption of solutions of known concentration is measured and a graph produced. ...
Kinetics and mechanism of macrocyclic complex
... conditions, dissociation of the complex to form the free ligand is rate-determining (ref 2 6 , 2 7 ) . As the dissociation reactions of macrocyclic complexes are often relatively slow, reactions may be monitored using conventional or stopped-flow techniques. The various kinetic methods have been use ...
... conditions, dissociation of the complex to form the free ligand is rate-determining (ref 2 6 , 2 7 ) . As the dissociation reactions of macrocyclic complexes are often relatively slow, reactions may be monitored using conventional or stopped-flow techniques. The various kinetic methods have been use ...
Chemical speciation of polynuclear complexes containing
... Acidic solutions of the metal ions were prepared from commercial salts and standardized according to standard techniques [25–27]. All the solutions were freed of carbon dioxide by boiling the solvent and subsequent cooling under Ar atmosphere. The standard HCl solution was prepared from Merck standa ...
... Acidic solutions of the metal ions were prepared from commercial salts and standardized according to standard techniques [25–27]. All the solutions were freed of carbon dioxide by boiling the solvent and subsequent cooling under Ar atmosphere. The standard HCl solution was prepared from Merck standa ...
Lecture 26
... Stoichiometry of Complexes A species that bonds to a metal cation to form a complex is known as a ligand. The number of ligands is called the coordination number) The stabilization of a metal complex by a ligand with more than one donor atom is known as the chelate effect. ...
... Stoichiometry of Complexes A species that bonds to a metal cation to form a complex is known as a ligand. The number of ligands is called the coordination number) The stabilization of a metal complex by a ligand with more than one donor atom is known as the chelate effect. ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.