Chm_451_F-12_Exam_3_..
... 22b. (1 pt) Give an example of a ligand-to-metal charge transfer. You can show this with an arrow on the diagram above. 22c. (3 pts) We categorized transition metal complexes into three scenarios, the third of which was those compounds that could have only 18 electrons because the t2g orbitals shown ...
... 22b. (1 pt) Give an example of a ligand-to-metal charge transfer. You can show this with an arrow on the diagram above. 22c. (3 pts) We categorized transition metal complexes into three scenarios, the third of which was those compounds that could have only 18 electrons because the t2g orbitals shown ...
CO ORDINATION COMPOUNDS
... number, coordination polyhedron, homoleptic and heteroleptic. Answer (i)Coordination entity: A coordination entity is an electrically charged radical or species carrying a positive or negative charge. In a coordination entity, the central atom or ion is surrounded by a suitable number of neutral mol ...
... number, coordination polyhedron, homoleptic and heteroleptic. Answer (i)Coordination entity: A coordination entity is an electrically charged radical or species carrying a positive or negative charge. In a coordination entity, the central atom or ion is surrounded by a suitable number of neutral mol ...
Encapsulated, Stabilized Alkali Metals for Birch
... are easy to handle in the lab, pilot plant, and commercial manufacturing facility. They are typically produced with loadings of up to 40 wt. % alkali metal. The powders can be utilized in batch and continuous processes at ambient temperatures and pressures and do not require the use of liquid ammoni ...
... are easy to handle in the lab, pilot plant, and commercial manufacturing facility. They are typically produced with loadings of up to 40 wt. % alkali metal. The powders can be utilized in batch and continuous processes at ambient temperatures and pressures and do not require the use of liquid ammoni ...
Ni complexes of redox-active pincers with pendant H-bonding sites
... certain that the complexes retain their structure in solution, although a 4-coordinate complex can be excluded because it would show an NMR spectrum. The multielectron ligand redox behavior of 2Zn suggests that two reducing equivalents could be hosted within the ligand framework without reduction of ...
... certain that the complexes retain their structure in solution, although a 4-coordinate complex can be excluded because it would show an NMR spectrum. The multielectron ligand redox behavior of 2Zn suggests that two reducing equivalents could be hosted within the ligand framework without reduction of ...
[Fe(L)Ci2]
... FeCIz.4H20 (1.00g/5.03 mmol), diethylenetriamine (1.04g/10.08 mmol) and malonic acid (1.05g/10.08 mmol). The compound was recrystallized in benzene. Synthesis of 2,5,13,16-tetraoxo-l,6,12,17-tetrazaeyelodidodeeane iron(ll) chloride [Fe(L6)CI2] This preparation was analogous to the above. Succinic ac ...
... FeCIz.4H20 (1.00g/5.03 mmol), diethylenetriamine (1.04g/10.08 mmol) and malonic acid (1.05g/10.08 mmol). The compound was recrystallized in benzene. Synthesis of 2,5,13,16-tetraoxo-l,6,12,17-tetrazaeyelodidodeeane iron(ll) chloride [Fe(L6)CI2] This preparation was analogous to the above. Succinic ac ...
the PDF file
... formed when the carbonyl carbon donates a lone pair of electrons to the vacant orbital of the metal. A π bond is formed by the donation of a pair of electrons from the filled metal d orbital into the vacant anti-bonding π* orbital (also known as back bonding of the carbonyl group). The σ bond streng ...
... formed when the carbonyl carbon donates a lone pair of electrons to the vacant orbital of the metal. A π bond is formed by the donation of a pair of electrons from the filled metal d orbital into the vacant anti-bonding π* orbital (also known as back bonding of the carbonyl group). The σ bond streng ...
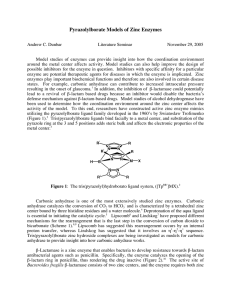
Pyrazolylborate Models of Zinc Enzymes
... enzyme are potential therapeutic agents for diseases in which the enzyme is implicated. Zinc enzymes play important biochemical functions and therefore are also involved in certain disease states. For example, carbonic anhydrase can contribute to increased intraocular pressure resulting in the onset ...
... enzyme are potential therapeutic agents for diseases in which the enzyme is implicated. Zinc enzymes play important biochemical functions and therefore are also involved in certain disease states. For example, carbonic anhydrase can contribute to increased intraocular pressure resulting in the onset ...
Study of η6 - cyclic π-perimeter hydrocarbon ruthenium complexes
... 6), as their (complexes 1–4, 6) PF6 salt or (complex 5) BF4 salt. The complexes were obtained by treatment of respective precursors, [(η6 -arene)Ru(μ-Cl)Cl]2 (arene = C6 H6 , p-i PrC6 H4 Me, C6 Me6 ) in 1:2 and 1:1 molar ratio with pppdH and dppdH in the presence of NH4 PF6 /NH4 BF4 . All the comple ...
... 6), as their (complexes 1–4, 6) PF6 salt or (complex 5) BF4 salt. The complexes were obtained by treatment of respective precursors, [(η6 -arene)Ru(μ-Cl)Cl]2 (arene = C6 H6 , p-i PrC6 H4 Me, C6 Me6 ) in 1:2 and 1:1 molar ratio with pppdH and dppdH in the presence of NH4 PF6 /NH4 BF4 . All the comple ...
Communications to the Editor - UCLA Chemistry and Biochemistry
... In reality, a 1:l mixture of the two alcohols 12a and 12b was produced upon catalytic hydrogenation over a rhodium-onalumina catalyst. The use of other catalysts and/or solvents," as well as attempts at dissolving metal reduction,12gave mainly products resulting from hydrogenolysis of the allylic al ...
... In reality, a 1:l mixture of the two alcohols 12a and 12b was produced upon catalytic hydrogenation over a rhodium-onalumina catalyst. The use of other catalysts and/or solvents," as well as attempts at dissolving metal reduction,12gave mainly products resulting from hydrogenolysis of the allylic al ...
211 - Chimica
... The reactions of the spacers L1–L5 with Co(NO3)2ⴢ6H2O were carried out in different solvent systems using various molar ratios of the reagents from 1 : 1 to 2 : 1. Crystalline products were isolated with all the ligands, except for L1. We have also observed the formation of different products with L5 ...
... The reactions of the spacers L1–L5 with Co(NO3)2ⴢ6H2O were carried out in different solvent systems using various molar ratios of the reagents from 1 : 1 to 2 : 1. Crystalline products were isolated with all the ligands, except for L1. We have also observed the formation of different products with L5 ...
Iron-only hydrogenase: Synthetic, structural and reactivity studies of
... weak magnetic coupling of the paramagnetic {4Fe4S} cluster to the subsite is observed [32,34]. Thus the spin-density in the subsite resides on the distal Fe-atom. The question is whether this metal atom has a formal oxidation state of Fe(III) or is in a biologically unprecedented Fe(I) state. A Mös ...
... weak magnetic coupling of the paramagnetic {4Fe4S} cluster to the subsite is observed [32,34]. Thus the spin-density in the subsite resides on the distal Fe-atom. The question is whether this metal atom has a formal oxidation state of Fe(III) or is in a biologically unprecedented Fe(I) state. A Mös ...
Review of selective separations of cobalt, uranium, zinc, nickel and
... feed solution is passed through the column and the target ion is removed from the solution. SuperLig® products are designed to bind selectively with ions based on multiple parameters such as size, coordination chemistry, and geometry. In contrast, conventional separation methods such as precipitatio ...
... feed solution is passed through the column and the target ion is removed from the solution. SuperLig® products are designed to bind selectively with ions based on multiple parameters such as size, coordination chemistry, and geometry. In contrast, conventional separation methods such as precipitatio ...
Transition metal sulfur chemistry and its relevance to
... The comparison of reactions (1) and (2) raises the question: Why does the tungsten complex react by purely ligand redox while the molybdenum complex undergoes an internal electron transfer process? Clearly, molybdenum is generally more easily reduced than is tungsten. One manifestation of this trend ...
... The comparison of reactions (1) and (2) raises the question: Why does the tungsten complex react by purely ligand redox while the molybdenum complex undergoes an internal electron transfer process? Clearly, molybdenum is generally more easily reduced than is tungsten. One manifestation of this trend ...
Common Structural Features in Calcium
... variable coordination modes have been reported.14 Some of these materials could be synthesized as single crystals,11 demonstrating their potential for crystal engineering. From previous studies, it was apparent that solids with low dimensionality tend to crystallize at room temperature and low pH, w ...
... variable coordination modes have been reported.14 Some of these materials could be synthesized as single crystals,11 demonstrating their potential for crystal engineering. From previous studies, it was apparent that solids with low dimensionality tend to crystallize at room temperature and low pH, w ...
Metal Saccharinates and Their Complexes with N
... by other authors are also included in this analysis. In order to obtain information on the geometry of deprotonated saccharin in the solid state we carried out a survey of the Cambridge Structural Database (CSD) for crystal structures of saccharinates.5 In addition, we performed geometry optimizatio ...
... by other authors are also included in this analysis. In order to obtain information on the geometry of deprotonated saccharin in the solid state we carried out a survey of the Cambridge Structural Database (CSD) for crystal structures of saccharinates.5 In addition, we performed geometry optimizatio ...
lecture 7 complexation
... For quantitative analysis, as usual, a large Kf is desired Monodentate chelating agents are not used for quantitative analysis (no sharp endpoint) ...
... For quantitative analysis, as usual, a large Kf is desired Monodentate chelating agents are not used for quantitative analysis (no sharp endpoint) ...
No Slide Title
... Electron Paramagnetic Resonance (EPR) is an important tool in experimental studies of systems containing unpaired electrons[1]. The traditional application areas for EPR include studies of transition metal complexes, stable organic radicals, transient reaction intermediates, as well as solid state a ...
... Electron Paramagnetic Resonance (EPR) is an important tool in experimental studies of systems containing unpaired electrons[1]. The traditional application areas for EPR include studies of transition metal complexes, stable organic radicals, transient reaction intermediates, as well as solid state a ...
d_block - ilc.edu.hk
... In the middle of the series more electrons enter the inner 3d sub-shell The inner 3d electrons shield the outer 4s electrons effectively the effective nuclear charge experienced by 4s electrons increases very slowly only a slow decrease in atomic radius in this region ...
... In the middle of the series more electrons enter the inner 3d sub-shell The inner 3d electrons shield the outer 4s electrons effectively the effective nuclear charge experienced by 4s electrons increases very slowly only a slow decrease in atomic radius in this region ...
Transition Metals and Coordination Chemistry
... How many of the following coordination compounds will form a precipitate when treated with an aqueous solution of AgNO3? [CrCl3(NH3)3] [Cr(NH3)6]Cl3 [CrCl(NH3)5](OH)2 Na3[Cr(CN)6] a) 0 b) 1 c) 2 ...
... How many of the following coordination compounds will form a precipitate when treated with an aqueous solution of AgNO3? [CrCl3(NH3)3] [Cr(NH3)6]Cl3 [CrCl(NH3)5](OH)2 Na3[Cr(CN)6] a) 0 b) 1 c) 2 ...
Chapter 19: Phenomena
... Note: If the ligand already contains a Greek prefix (ethylenediamine) or if it is polydentate (able to attach at more than one binding site) then use the following prefixes instead. In addition, the name of the ligand is put in parenthesis. ...
... Note: If the ligand already contains a Greek prefix (ethylenediamine) or if it is polydentate (able to attach at more than one binding site) then use the following prefixes instead. In addition, the name of the ligand is put in parenthesis. ...
Aldehydes and ketones
... • Neither aldehydes nor ketones possess the ability to H-bond with other molecules like themselves. Consequently, boiling points for aldehydes and ketones are lower than for alcohols of similar molar mass. • The C-O double bond in these molecules is polar, so dipoledipole forces do exist. As a resul ...
... • Neither aldehydes nor ketones possess the ability to H-bond with other molecules like themselves. Consequently, boiling points for aldehydes and ketones are lower than for alcohols of similar molar mass. • The C-O double bond in these molecules is polar, so dipoledipole forces do exist. As a resul ...
Carbonyl Condensation Reactions
... conjugated enone as it is formed, especially if reaction conditions are pushed, eg high temperature. Mixed or "crossed" Aldol Reactions — If two different carbonyl compounds are allowed to react in an aldol reaction four products usually result; each carbonyl compound forms an enolate and each enola ...
... conjugated enone as it is formed, especially if reaction conditions are pushed, eg high temperature. Mixed or "crossed" Aldol Reactions — If two different carbonyl compounds are allowed to react in an aldol reaction four products usually result; each carbonyl compound forms an enolate and each enola ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.





![[Fe(L)Ci2]](http://s1.studyres.com/store/data/017913694_1-e50c65b89c5a2b87b0eddc6dd9f4061a-300x300.png)