1 slide per page() - Wayne State University Physics and Astronomy
... electrons, when an electron moves from the n = 1 level to the n = 3 level, the circumference of its orbit becomes 9 times greater. This occurs because (a) there are 3 times as many wavelengths in the new orbit, (b) there are 3 times as many wavelengths and each wavelength is 3 times as long, (c) the ...
... electrons, when an electron moves from the n = 1 level to the n = 3 level, the circumference of its orbit becomes 9 times greater. This occurs because (a) there are 3 times as many wavelengths in the new orbit, (b) there are 3 times as many wavelengths and each wavelength is 3 times as long, (c) the ...
Fundamental processes: Atomic Physics
... Number of shells – in presence of levels closer to nucleus outermost electrons are further from nucleus and are not so strongly adracted Shielding – electrons on orbits closer to nucleus shield/protect outermost electron from adracIon of nucleus ...
... Number of shells – in presence of levels closer to nucleus outermost electrons are further from nucleus and are not so strongly adracted Shielding – electrons on orbits closer to nucleus shield/protect outermost electron from adracIon of nucleus ...
Atomic Emission Spectrometry - San Diego Unified School District
... The electrons in an atom occupy different energy levels, as you know. When all of the electrons are at the lowest possible energy level they are said to be in the ground state. Electrons do not always stay in the ground state. Sometimes they can be promoted to a higher-energy electron shell. This ca ...
... The electrons in an atom occupy different energy levels, as you know. When all of the electrons are at the lowest possible energy level they are said to be in the ground state. Electrons do not always stay in the ground state. Sometimes they can be promoted to a higher-energy electron shell. This ca ...
ap chemistry chapter 8 bonding
... • Ionic bonds form when an atom that loses electrons easily reacts with an atom that has a high affinity for electrons. The charged ions are held together by their mutual attraction. • Ionic bonds form because the ion pair has lower energy than the separated ions. All bonds form in order to reach a ...
... • Ionic bonds form when an atom that loses electrons easily reacts with an atom that has a high affinity for electrons. The charged ions are held together by their mutual attraction. • Ionic bonds form because the ion pair has lower energy than the separated ions. All bonds form in order to reach a ...
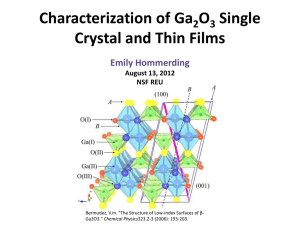
Characterization of Ga 2 0 3 Single Crystal and Thin Films
... Bermudez, V.m. "The Structure of Low-index Surfaces of βGa2O3." Chemical Physics323.2-3 (2006): 193-203. ...
... Bermudez, V.m. "The Structure of Low-index Surfaces of βGa2O3." Chemical Physics323.2-3 (2006): 193-203. ...
On the use of ERL for gamma production
... wavelength 1 micron and undulator period u = 10 cm, one get electron energy about Emc2 1 K 2 u 200MeV (for the undulator parameter K slightly more than 1). Then for the charge per bunch Q = 5 nC the bunch energy is QE/e = 1 J. The typical FEL efficiency is 1%. It means, that energy extracti ...
... wavelength 1 micron and undulator period u = 10 cm, one get electron energy about Emc2 1 K 2 u 200MeV (for the undulator parameter K slightly more than 1). Then for the charge per bunch Q = 5 nC the bunch energy is QE/e = 1 J. The typical FEL efficiency is 1%. It means, that energy extracti ...
Slayt 1
... Consider the elements K and I. What are their group numbers? How many electrons are there in their outer energy levels? K and I for the compound KI. K loses one electron K+ I gains one electron IWhat is the total charge of the compound KI? ...
... Consider the elements K and I. What are their group numbers? How many electrons are there in their outer energy levels? K and I for the compound KI. K loses one electron K+ I gains one electron IWhat is the total charge of the compound KI? ...
Conductivities and transmission coefficients of ultra-thin disordered metallic films B. J.
... The calculated conductivity σ increases almost linearly with increasing film thickness Lz. This is somewhat surprising, because σ is a material constant and should not depend on the size of the sample. In our model of quasi-two-dimensional electron gas, however, an increase of Lz gives rise to an in ...
... The calculated conductivity σ increases almost linearly with increasing film thickness Lz. This is somewhat surprising, because σ is a material constant and should not depend on the size of the sample. In our model of quasi-two-dimensional electron gas, however, an increase of Lz gives rise to an in ...
Light, Energy, and More
... Four d orbitals have same shape but different orientations Fifth d orbital, 3dz2 is shaped and oriented different from the other four ...
... Four d orbitals have same shape but different orientations Fifth d orbital, 3dz2 is shaped and oriented different from the other four ...
General Chemistry: An Integrated Approach
... having equal velocities is brought into a magnetic field, the lightest ions are deflected the most, making a tighter circle ...
... having equal velocities is brought into a magnetic field, the lightest ions are deflected the most, making a tighter circle ...
The Quantum-Mechanical Model of the Atom
... Each packet or bundle of energy is called a quantum. A fraction of a quantum is never emitted. A quantum is the smallest amount of energy that can be emitted or absorbed in the form of electromagnetic radiation. ...
... Each packet or bundle of energy is called a quantum. A fraction of a quantum is never emitted. A quantum is the smallest amount of energy that can be emitted or absorbed in the form of electromagnetic radiation. ...
Grade 10 Science – Unit 2
... Lewis Dot Diagrams are very useful. With them, you can (1) determine the type(s) of covalent bonds that an element may make, and (2) predict the type of ion that an atom might make. Each dot diagram consists of an elemental symbol (called the kernel) and a group of 1-8 dots which shows the configura ...
... Lewis Dot Diagrams are very useful. With them, you can (1) determine the type(s) of covalent bonds that an element may make, and (2) predict the type of ion that an atom might make. Each dot diagram consists of an elemental symbol (called the kernel) and a group of 1-8 dots which shows the configura ...
Chemistry Unit IV – The Electron
... of particles he called photons. 3. A __________________ is a quantum of light – a particle of radiation. a. Has __________________ rest mass and carries a quantum of energy. b. 4. According to this theory proposed by Einstein, radiation is absorbed and emitted only in whole numbers of photons. 5. Di ...
... of particles he called photons. 3. A __________________ is a quantum of light – a particle of radiation. a. Has __________________ rest mass and carries a quantum of energy. b. 4. According to this theory proposed by Einstein, radiation is absorbed and emitted only in whole numbers of photons. 5. Di ...
Notes on Atomic Structure 1. Introduction 2. Hydrogen Atoms and
... The sum of the energies of the two emitted photons is E2s−E1s = 10.2 eV. The photons have a continuous spectrum since there is no other constraint on their energies. This is a major contri ...
... The sum of the energies of the two emitted photons is E2s−E1s = 10.2 eV. The photons have a continuous spectrum since there is no other constraint on their energies. This is a major contri ...
New Bohr model calculates Helium ground state energy
... Fig.3: The electrons have moved one quarter of their orbitals. Here we investigate how the electrons of the helium atom are moving by calculating the Coulomb force among the two electrons and the nucleus at short time intervals. Methods and results: The computer program (class filename: MathMethod) w ...
... Fig.3: The electrons have moved one quarter of their orbitals. Here we investigate how the electrons of the helium atom are moving by calculating the Coulomb force among the two electrons and the nucleus at short time intervals. Methods and results: The computer program (class filename: MathMethod) w ...
Energy-Angle Distribution of Thin Target Bremsstrahlung
... E, and k are all large in comparison with p, and only leading terms are retained. Since most of the radiation comes off at angles 80~ p/Eo, it turns out that neither calculation is valid for angles large in comparison with IJ/Eo. The large angle distribution has been treated by Hough, 7 and makes on ...
... E, and k are all large in comparison with p, and only leading terms are retained. Since most of the radiation comes off at angles 80~ p/Eo, it turns out that neither calculation is valid for angles large in comparison with IJ/Eo. The large angle distribution has been treated by Hough, 7 and makes on ...
File
... rays which travel in straight lines. Euclid was the first to develop a detailed theory of reflection using such rays. Nobody was able to develop theories of light much further as more intricate experimentation was not possible. At the beginning of the 11th century the Arabic scientist Alhazen develo ...
... rays which travel in straight lines. Euclid was the first to develop a detailed theory of reflection using such rays. Nobody was able to develop theories of light much further as more intricate experimentation was not possible. At the beginning of the 11th century the Arabic scientist Alhazen develo ...
Energy Level Models - Middle School Chemistry
... atoms. Some middle school texts show the electrons in pairs on an energy level. This pairing of electrons is intended to suggest information about the substructure within energy levels. This substructure is made up of regions called orbitals which comprise each energy level. The shape and size of th ...
... atoms. Some middle school texts show the electrons in pairs on an energy level. This pairing of electrons is intended to suggest information about the substructure within energy levels. This substructure is made up of regions called orbitals which comprise each energy level. The shape and size of th ...
Chemistry 2202 Background Information – Chapter 1 (pg
... sand, clay, silt, decaying organism and leaves Heterogeneous – mixtures in which different components are clearly visible Homogeneous – mixtures which appear to be just one substance Pure substance - having a definite composition which stays the same in response to physical changes. Ex. Copper, ...
... sand, clay, silt, decaying organism and leaves Heterogeneous – mixtures in which different components are clearly visible Homogeneous – mixtures which appear to be just one substance Pure substance - having a definite composition which stays the same in response to physical changes. Ex. Copper, ...
Auger electron spectroscopy
.jpg?width=300)
Auger electron spectroscopy (AES; pronounced [oʒe] in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.