Lewis Structure Activity
... Name ______________________________________________________________ Pd ____ ...
... Name ______________________________________________________________ Pd ____ ...
The influence of effective mass on magnetoresistance in ultrathin Fe/Cr/Fe films K. W
... resistance observed when rotating from an antiparallel to parallel alignment of film magnetizations. For its description, two different approaches are usually used: the quasi-classical method based on the Boltzmann equation [3–5] or the quantum -mechanical Kubo formalism [6]. Most of models are idea ...
... resistance observed when rotating from an antiparallel to parallel alignment of film magnetizations. For its description, two different approaches are usually used: the quasi-classical method based on the Boltzmann equation [3–5] or the quantum -mechanical Kubo formalism [6]. Most of models are idea ...
Electron—Proton Twins, Orderly Arranged in The Inside of Bioatoms
... At an angular momentum with u 3.84 104 m s the two protons come together since D/Dp = 0 → D = 0 At an angular momentum with u 1.92 104 m s a rupture of Coulomb’s barrier occurs. Now the protons balance between them at a contact position. At an angular momentum with velocities of the pos ...
... At an angular momentum with u 3.84 104 m s the two protons come together since D/Dp = 0 → D = 0 At an angular momentum with u 1.92 104 m s a rupture of Coulomb’s barrier occurs. Now the protons balance between them at a contact position. At an angular momentum with velocities of the pos ...
lecture1
... • In electrons shorter wavelength electrons are more energetic and have a longer focal length than longer wavelength electrons. ...
... • In electrons shorter wavelength electrons are more energetic and have a longer focal length than longer wavelength electrons. ...
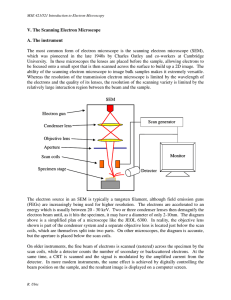
V. The Scanning Electron Microscope A. The instrument The most
... In order to resolve two points on a specimen, there must be a visible difference in the signal generated from them. If the average number of electrons detected from one point is n , then statistically that number will vary by up to n about the mean. Natural contrast, C, is defined as the normalized ...
... In order to resolve two points on a specimen, there must be a visible difference in the signal generated from them. If the average number of electrons detected from one point is n , then statistically that number will vary by up to n about the mean. Natural contrast, C, is defined as the normalized ...
Lecture 13 - UD Physics
... The expectation values Dik for all possible transitions between arbitrary levels i , k = 1, 2, . . . , n can be arranged in an n × n matrix. The Dik are therefore called electric-dipole matrix elements. If some of the matrix elements are zero, the corresponding transition does not occur. One says th ...
... The expectation values Dik for all possible transitions between arbitrary levels i , k = 1, 2, . . . , n can be arranged in an n × n matrix. The Dik are therefore called electric-dipole matrix elements. If some of the matrix elements are zero, the corresponding transition does not occur. One says th ...
chemistry 101 spring 2002 part 1
... Directions: (1) Put your name, S.I.D. number and signature on the free response part of the exam where indicated. (2) Each multiple choice question is actually 2 questions on your scanning sheet. If you are sure of an answer, put the same answer down for both questions for 5 pts. If you cannot decid ...
... Directions: (1) Put your name, S.I.D. number and signature on the free response part of the exam where indicated. (2) Each multiple choice question is actually 2 questions on your scanning sheet. If you are sure of an answer, put the same answer down for both questions for 5 pts. If you cannot decid ...
Lecture 12 Atomic structure
... Since single-particle Hamiltonian Ĥ0 continues to commute with the angular momentum operator, [Ĥ0 , L̂] = 0, its eigenfunctions remain indexed by quantum numbers (n, #, m! , ms ). However, since effective potential, V (r ) + Ui (r ), is no longer Coulomb-like, # values for a given n need not be de ...
... Since single-particle Hamiltonian Ĥ0 continues to commute with the angular momentum operator, [Ĥ0 , L̂] = 0, its eigenfunctions remain indexed by quantum numbers (n, #, m! , ms ). However, since effective potential, V (r ) + Ui (r ), is no longer Coulomb-like, # values for a given n need not be de ...
the zeeman effect
... additional energy just as a bar magnet does and consequently the original energy level is shifted. The energy shift may be positive, zero, or even negative, depending on the angle between the electron magnetic dipole moment and the field. Due to Zeeman effect, some degenerate energy levels will spli ...
... additional energy just as a bar magnet does and consequently the original energy level is shifted. The energy shift may be positive, zero, or even negative, depending on the angle between the electron magnetic dipole moment and the field. Due to Zeeman effect, some degenerate energy levels will spli ...
Basic Chemistry Lecture Notes - Roderick Biology
... • Electrons can be found on electron shells • Electrons on the outermost electron shell are called valence electrons Valence Electron e- ...
... • Electrons can be found on electron shells • Electrons on the outermost electron shell are called valence electrons Valence Electron e- ...
Document
... There are seven base units, and each of these base units are nominally dimensionally independent. length(m), mass(kg), time(s), temperature(K), mole(mol), (electric current(A), Luminous intensity(cd)) From these seven base units, several other units are derived, which means the derived units can be ...
... There are seven base units, and each of these base units are nominally dimensionally independent. length(m), mass(kg), time(s), temperature(K), mole(mol), (electric current(A), Luminous intensity(cd)) From these seven base units, several other units are derived, which means the derived units can be ...
Chapter 7: ELECTRONS IN ATOMS AND PERIODIC PROPERTIES
... amplitude. The “square” of a wavefunction gives the probability density…the likelihood of finding the particle in region of space. • The wavefunctions and kinetic energies available to a quantum particle are quantized if the particle is subject to a constraining potential. • We can determine the wav ...
... amplitude. The “square” of a wavefunction gives the probability density…the likelihood of finding the particle in region of space. • The wavefunctions and kinetic energies available to a quantum particle are quantized if the particle is subject to a constraining potential. • We can determine the wav ...
Title Near-ultraviolet inverse photoemission spectroscopy using ultra
... centered at 3.71 eV with w = 0.11 eV gives overall resolution of 0.27 eV in FWHM. Since the values are close to E = 0.25 eV, the overall resolution is considered to be limited by the energy spread of electron rather than the resolution of band pass filters. ...
... centered at 3.71 eV with w = 0.11 eV gives overall resolution of 0.27 eV in FWHM. Since the values are close to E = 0.25 eV, the overall resolution is considered to be limited by the energy spread of electron rather than the resolution of band pass filters. ...
che-20028 QC lecture 1 - Rob Jackson`s Website
... causes electrons to be emitted. • Measure the energy of the emitted electrons, by applying a voltage across the cell in the opposite direction to balance the voltage of the emitted electrons (using ½ mv2=Ve) CHE-20028 QC lecture 1 ...
... causes electrons to be emitted. • Measure the energy of the emitted electrons, by applying a voltage across the cell in the opposite direction to balance the voltage of the emitted electrons (using ½ mv2=Ve) CHE-20028 QC lecture 1 ...
Low Dose X-Ray Radiation Source for Angiography Based on
... excite the characteristic X-ray of La or Ba emitters having considerable photon flux. Channeling radiation source, one of the most powerful radiation emitters by relativistic electrons in crystals is discussed below as a possible alternative of these techniques [1]. For the angiography and radiograp ...
... excite the characteristic X-ray of La or Ba emitters having considerable photon flux. Channeling radiation source, one of the most powerful radiation emitters by relativistic electrons in crystals is discussed below as a possible alternative of these techniques [1]. For the angiography and radiograp ...
Single-photon multiple ionization processes studied by electron coincidence spectroscopy Per Linusson
... In this context the main topic of this thesis can be understood, which is single-photon multiple ionization. Excluding the well-known Auger effect the emission of two or more electrons induced by just one photon is an important manifestation of electron correlations. Unfortunately conventional photo ...
... In this context the main topic of this thesis can be understood, which is single-photon multiple ionization. Excluding the well-known Auger effect the emission of two or more electrons induced by just one photon is an important manifestation of electron correlations. Unfortunately conventional photo ...
Chapter 7 - Suffolk County Community College
... Max Planck (1858-1947) proposed that light waves existed as discrete packets of energy, “quanta” in order to account for the “ultraviolet catastrophe” predicted by classical physics. The “ultraviolet catastrophe” arises from the classical theory for the energy emitted by an ideal blackbody governed ...
... Max Planck (1858-1947) proposed that light waves existed as discrete packets of energy, “quanta” in order to account for the “ultraviolet catastrophe” predicted by classical physics. The “ultraviolet catastrophe” arises from the classical theory for the energy emitted by an ideal blackbody governed ...
Auger electron spectroscopy
.jpg?width=300)
Auger electron spectroscopy (AES; pronounced [oʒe] in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.