Question paper - Unit A173/02 - Module C7 - Higher tier
... Scientists are investigating a new catalyst. The new catalyst is an enzyme. Here is some information about both catalysts. ...
... Scientists are investigating a new catalyst. The new catalyst is an enzyme. Here is some information about both catalysts. ...
Ch 8 Notes: Chemical Equations and Reactions
... Endothermic Reactions – when energy is absorbed or taken in during a chemical reaction. Energy is required when a compound is decomposed or breaks down; energy is a reactant and is written on the left of the arrow: 2H2O + energy 2H2 + O2 B. ...
... Endothermic Reactions – when energy is absorbed or taken in during a chemical reaction. Energy is required when a compound is decomposed or breaks down; energy is a reactant and is written on the left of the arrow: 2H2O + energy 2H2 + O2 B. ...
GCSE_C2_Revision_+_Exam_Questions
... that lose electrons become positively charged ions. Atoms that gain electrons become negatively charged ions. Ions have the electronic structure of a noble gas (Group 0). The elements in Group 1 of the periodic table, the alkali metals, have similar chemical properties. They all react with non-metal ...
... that lose electrons become positively charged ions. Atoms that gain electrons become negatively charged ions. Ions have the electronic structure of a noble gas (Group 0). The elements in Group 1 of the periodic table, the alkali metals, have similar chemical properties. They all react with non-metal ...
metal-water interactions and hydrogen bond strength
... HDO molecules (2568, 2520 and 2334 cm-1, ambient temperature) which shift to lower frequencies on cooling. Furthermore, the band at the lowest wavenumber transforms into two bands at 2282 and 2212 cm-1 (liquid nitrogen temperature, see Fig. 4). The spectroscopic experiments allow us to deduce the ex ...
... HDO molecules (2568, 2520 and 2334 cm-1, ambient temperature) which shift to lower frequencies on cooling. Furthermore, the band at the lowest wavenumber transforms into two bands at 2282 and 2212 cm-1 (liquid nitrogen temperature, see Fig. 4). The spectroscopic experiments allow us to deduce the ex ...
File
... Chemists generally refer to the energy given out when a fuel burns in kJmol-1 because this compares the same number of molecules of each fuel. For use as fuels it is sometimes better to convert the units from kJmol-1 to kJg-1 (OR the energy density) of a fuel ...
... Chemists generally refer to the energy given out when a fuel burns in kJmol-1 because this compares the same number of molecules of each fuel. For use as fuels it is sometimes better to convert the units from kJmol-1 to kJg-1 (OR the energy density) of a fuel ...
Final Preparation
... 58. The function of the enzyme-substrate complex is to provide an alternative reaction pathway that A) lowers the energy of the products B) changes the concentration of the substrate C) lowers the energy of the substrate D) decreases the activation energy for the reaction E) changes the possible pro ...
... 58. The function of the enzyme-substrate complex is to provide an alternative reaction pathway that A) lowers the energy of the products B) changes the concentration of the substrate C) lowers the energy of the substrate D) decreases the activation energy for the reaction E) changes the possible pro ...
Bal Equations notes.cwk (WP)
... We cannot change the way compounds are put together but we can adjust the number of compounds that are made. For example: Magnesium reacts with oxygen to form magnesium oxide. Since oxygen is a diatomic molecule we can only obtain them in groups of two atoms. We know that the formula for magnesium o ...
... We cannot change the way compounds are put together but we can adjust the number of compounds that are made. For example: Magnesium reacts with oxygen to form magnesium oxide. Since oxygen is a diatomic molecule we can only obtain them in groups of two atoms. We know that the formula for magnesium o ...
Precipitation Reactions
... towards making solutions of soluble salts, and a route toward making pure elemental metals. Example: How could we synthesize a solution of aluminum chlorate? Start with a solution of metal chlorate (where the metal is less active than aluminum) and then add ...
... towards making solutions of soluble salts, and a route toward making pure elemental metals. Example: How could we synthesize a solution of aluminum chlorate? Start with a solution of metal chlorate (where the metal is less active than aluminum) and then add ...
CHM 101 - Academic Computer Center
... Cold packs, whose temperatures are lowered when ammonium nitrate dissolves in water, are carried by athletic trainers when transporting ice is not possible. Which of the following is true of this reaction? A. H < 0, process is exothermic B. H > 0, process is exothermic C. H < 0, process is endoth ...
... Cold packs, whose temperatures are lowered when ammonium nitrate dissolves in water, are carried by athletic trainers when transporting ice is not possible. Which of the following is true of this reaction? A. H < 0, process is exothermic B. H > 0, process is exothermic C. H < 0, process is endoth ...
Chapter 14 Chemical Reactions
... 14.1 Balancing chemical equations A balanced chemical equation has the same number of each type of atom on the product side and the reactant side. To balance the equation, we add another water molecule to the product side and add another oxygen molecule to the reactant side. ...
... 14.1 Balancing chemical equations A balanced chemical equation has the same number of each type of atom on the product side and the reactant side. To balance the equation, we add another water molecule to the product side and add another oxygen molecule to the reactant side. ...
chemical*equations
... Hydrogen'and'Oxygen'react'vigorously'to'form'water.'If' 275'hydrogen'molecules'are'reacted'with'125'oxygen' molecules'in'a'closed'container,'how'many'hydrogen,' oxygen,'and'water'molecules'will'remain'after'the' reaction'is'complete?' (a)'150'hydrogen'+'0'Oxygen'+'125'water' (b)'0'hydrogen'+'25'oxyg ...
... Hydrogen'and'Oxygen'react'vigorously'to'form'water.'If' 275'hydrogen'molecules'are'reacted'with'125'oxygen' molecules'in'a'closed'container,'how'many'hydrogen,' oxygen,'and'water'molecules'will'remain'after'the' reaction'is'complete?' (a)'150'hydrogen'+'0'Oxygen'+'125'water' (b)'0'hydrogen'+'25'oxyg ...
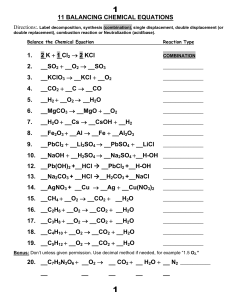
11 BALANCING CHEMICAL EQUATIONS 1. 2 K + 1
... The large numbers in front of chemical formulas. Coefficients represent the number of molecules of the substance in the reaction. ...
... The large numbers in front of chemical formulas. Coefficients represent the number of molecules of the substance in the reaction. ...
+ CuO Cu + O
... 9- Clear lime water turbid on passing ……………………………. Gas through it 10- On adding silver nitrate solution to sodium chloride solution, a …………………………… precipitate is formed with ……………………… colour. ...
... 9- Clear lime water turbid on passing ……………………………. Gas through it 10- On adding silver nitrate solution to sodium chloride solution, a …………………………… precipitate is formed with ……………………… colour. ...
AS specification - word format File
... k demonstrate an understanding that polarization of anions by cations leads to some covalency in an ionic bond, based on evidence from the Born-Haber cycle l use values calculated for standard heats of formation based on Born-Haber cycles to explain why particular ionic compounds exist, eg the relat ...
... k demonstrate an understanding that polarization of anions by cations leads to some covalency in an ionic bond, based on evidence from the Born-Haber cycle l use values calculated for standard heats of formation based on Born-Haber cycles to explain why particular ionic compounds exist, eg the relat ...
SAMPLE QUESTION PAPER-II Chemistry (Theory) Class-XII
... ionization enthalpies of nickel and platinum are: iH1 + iH2 (kJ mol-1) ...
... ionization enthalpies of nickel and platinum are: iH1 + iH2 (kJ mol-1) ...
precipitation rxn_level_packet
... Directions for the following 4 reactions: a. In one well of a well-plate, add three drops of each substance. b. Write down your observations for the reactants above. c. In parenthesis provided above, indicate if the product is soluble with an “aq” or forms a precipitate (solid) with an “s.” 1. Write ...
... Directions for the following 4 reactions: a. In one well of a well-plate, add three drops of each substance. b. Write down your observations for the reactants above. c. In parenthesis provided above, indicate if the product is soluble with an “aq” or forms a precipitate (solid) with an “s.” 1. Write ...
Dalton`s Laws worksheet
... Dalton’s Atomic Theory of Matter 1. Which of the following statements is part of Dalton’s atomic theory of matter? a. All atoms are identical b. All atoms of a given element are identical c. All atoms differ from one another d. Atoms of the same element can have a different shape 2. Dalton suggested ...
... Dalton’s Atomic Theory of Matter 1. Which of the following statements is part of Dalton’s atomic theory of matter? a. All atoms are identical b. All atoms of a given element are identical c. All atoms differ from one another d. Atoms of the same element can have a different shape 2. Dalton suggested ...
UNIT 1 - MATTER AND CHEMICAL BONDING
... c) isotopic abundance & relative atomic mass d) empirical & molecular formula e) law of definite proportions or constant composition f) quantitative relationships in a balanced equation g) limiting reagent h) actual yield, theoretical yield, percentage yield 2. A sample of glucose (C6H12O6) has a ma ...
... c) isotopic abundance & relative atomic mass d) empirical & molecular formula e) law of definite proportions or constant composition f) quantitative relationships in a balanced equation g) limiting reagent h) actual yield, theoretical yield, percentage yield 2. A sample of glucose (C6H12O6) has a ma ...
chemistry important question i
... What does the designation ‘6,6’ in nylon 6, 6 polymer mean? (b) Which polymer is obtained when free radical polymerisation of chloroprene occurs? Write the structure of the polymer thus obtained. An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the c ...
... What does the designation ‘6,6’ in nylon 6, 6 polymer mean? (b) Which polymer is obtained when free radical polymerisation of chloroprene occurs? Write the structure of the polymer thus obtained. An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the c ...
Catalytic Synthesis of Organophosphorus Compounds from
... largely on the substitutive P-O, P-C coupling of the high-valent phosphorus derivatives (PCl 3, PCl 5, POCl3) with an organic substrates. The emission of a large amount of hydrogen chloride accompanies these reactions with consequent serious environmental and corrosion problems. In this connection, ...
... largely on the substitutive P-O, P-C coupling of the high-valent phosphorus derivatives (PCl 3, PCl 5, POCl3) with an organic substrates. The emission of a large amount of hydrogen chloride accompanies these reactions with consequent serious environmental and corrosion problems. In this connection, ...
Introduction to Chemical Reactions and Equations Study Guide
... 2. The numbers written to the bottom right of element symbols in a compound are called ___subscripts________. These numbers tell you the ratio of __atoms______ in the compound. 3. Sometimes the names of ionic compounds include roman numerals written in parenthesis after the cation’s name. What do th ...
... 2. The numbers written to the bottom right of element symbols in a compound are called ___subscripts________. These numbers tell you the ratio of __atoms______ in the compound. 3. Sometimes the names of ionic compounds include roman numerals written in parenthesis after the cation’s name. What do th ...