C - Thierry Karsenti
... The module, Physical Chemistry 2, focuses on five (5) areas of physical chemistry important to many aspects of our lives: solutions, colloids, phase equilibrium, electrochemistry and nuclear chemistry. Solutions are often necessary to facilitate many chemical reactions in life processes or industry ...
... The module, Physical Chemistry 2, focuses on five (5) areas of physical chemistry important to many aspects of our lives: solutions, colloids, phase equilibrium, electrochemistry and nuclear chemistry. Solutions are often necessary to facilitate many chemical reactions in life processes or industry ...
FORMULA WRITNG
... 1) Write balanced equations (molecular, total ionic, and net ionic) for the reaction between each of the following solutions. If no reaction occurs, write “NR” for No Reaction. a. barium nitrate and sodium phosphate molecular: total ionic: net ionic: b. silver nitrate and sodium sulfide molecular: t ...
... 1) Write balanced equations (molecular, total ionic, and net ionic) for the reaction between each of the following solutions. If no reaction occurs, write “NR” for No Reaction. a. barium nitrate and sodium phosphate molecular: total ionic: net ionic: b. silver nitrate and sodium sulfide molecular: t ...
No Slide Title
... The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. ...
... The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. ...
Crystal Modelling
... Look at models A to J. Some are pictures or ideas. Others are things you can hold or try out. Work out which stage of X-ray crystallography each model helps to explain. Some stages have more than one model. Some models help explain more than one stage. Choose five or fewer models. Plan how to use th ...
... Look at models A to J. Some are pictures or ideas. Others are things you can hold or try out. Work out which stage of X-ray crystallography each model helps to explain. Some stages have more than one model. Some models help explain more than one stage. Choose five or fewer models. Plan how to use th ...
What is a Mineral?
... Are formed by natural processes. Have a definite volume and shape (it is a SOLID) Are elements or compounds with a unique chemical makeup Are made up of particles that are arranged in a pattern that is repeated over and over (called a CRYSTAL) ...
... Are formed by natural processes. Have a definite volume and shape (it is a SOLID) Are elements or compounds with a unique chemical makeup Are made up of particles that are arranged in a pattern that is repeated over and over (called a CRYSTAL) ...
Key Stage 3 – Crystal Modelling
... Look at models A to J. Some are pictures or ideas. Others are things you can hold or try out. Work out which stage of X-ray crystallography each model helps to explain. Some stages have more than one model. Some models help explain more than one stage. Choose five or fewer models. Plan how to use th ...
... Look at models A to J. Some are pictures or ideas. Others are things you can hold or try out. Work out which stage of X-ray crystallography each model helps to explain. Some stages have more than one model. Some models help explain more than one stage. Choose five or fewer models. Plan how to use th ...
Novel weak coordination to silylium ions: formation of nearly linear
... silane are absent from the experimental spectrum. It is of interest to compare the degree of silylium ion character developed in each of these species with that in closely related silyl species. The extent of downfield shift of the 29Si NMR resonances and the approach of the R3Sid+ moiety towards pl ...
... silane are absent from the experimental spectrum. It is of interest to compare the degree of silylium ion character developed in each of these species with that in closely related silyl species. The extent of downfield shift of the 29Si NMR resonances and the approach of the R3Sid+ moiety towards pl ...
Crystal structure of high-temperature
... wing five years about twenty various crystalline superconductors were obtained at different laboratories all over the world (Table 1). The total amount of superconducting compounds, including isostructural ones which differ greatly in their chemical composition, now exceeds 500-600 and their number ...
... wing five years about twenty various crystalline superconductors were obtained at different laboratories all over the world (Table 1). The total amount of superconducting compounds, including isostructural ones which differ greatly in their chemical composition, now exceeds 500-600 and their number ...
Physical Chemistry II
... The module, Physical Chemistry 2, focuses on five (5) areas of physical chemistry important to many aspects of our lives: solutions, colloids, phase equilibrium, electrochemistry and nuclear chemistry. Solutions are often necessary to facilitate many chemical reactions in lif e processes or industry ...
... The module, Physical Chemistry 2, focuses on five (5) areas of physical chemistry important to many aspects of our lives: solutions, colloids, phase equilibrium, electrochemistry and nuclear chemistry. Solutions are often necessary to facilitate many chemical reactions in lif e processes or industry ...
Volcanoes and Igneous Activity Earth
... •Unstable isotopes emit particles and energy in a process known as radioactive decay ...
... •Unstable isotopes emit particles and energy in a process known as radioactive decay ...
Topic 4 Chemistry of the Elements of the Main Group
... groups: Metals, Metalloids and non-Metals Non-metals are not electrical conductors, they are characterised by a high electronegativity value (over 2). The valence electrons of non-metals are strongly attracted to their positively charged nucleus and are not available to conduct electricity. Metals ...
... groups: Metals, Metalloids and non-Metals Non-metals are not electrical conductors, they are characterised by a high electronegativity value (over 2). The valence electrons of non-metals are strongly attracted to their positively charged nucleus and are not available to conduct electricity. Metals ...
Go Here For PPT
... Chemistry Review : An ELEMENT is determined by the number of PROTONS (+). IONS - Atoms where the number of ELECTRONS (-) have been added or subtracted. ISOTOPES - Atoms where the number of NEUTRONS have been added or subtracted. ...
... Chemistry Review : An ELEMENT is determined by the number of PROTONS (+). IONS - Atoms where the number of ELECTRONS (-) have been added or subtracted. ISOTOPES - Atoms where the number of NEUTRONS have been added or subtracted. ...
What is a Mineral?
... •List the physical properties to identify minerals •Describe how physical properties are used to identify minerals •List characteristics that gems have that make them different from and more valuable than minerals •List the conditions necessary for a mineral to be classified an ore •List the propert ...
... •List the physical properties to identify minerals •Describe how physical properties are used to identify minerals •List characteristics that gems have that make them different from and more valuable than minerals •List the conditions necessary for a mineral to be classified an ore •List the propert ...
5073 Chemistry (SPA)
... 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique at ...
... 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique at ...
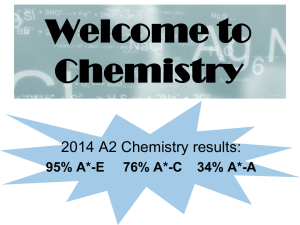
Welcome to Chemistry
... AS can be sat as a stand alone qualification over 1 year, exams are sat at the end of Y12. 2 written exams each 1 hour and 30 minutes. A level is the full 2 year qualification with all the exams at the end of Y13. 3 written papers each 2 hours. ...
... AS can be sat as a stand alone qualification over 1 year, exams are sat at the end of Y12. 2 written exams each 1 hour and 30 minutes. A level is the full 2 year qualification with all the exams at the end of Y13. 3 written papers each 2 hours. ...
111 Exam I Outline
... Analysis of nitrogen showed that the compound contains 46.62 % by mass nitrogen. The molar mass of the compound is about 170 + 15 g/mole. a) Calculate the empirical formula of the compound. 6a) Ans: C2H8N3O b) What is the molecular formula of the compound? 6b) Ans: C4H16N6O2 7) 169 g FeCr2O4, 298 g ...
... Analysis of nitrogen showed that the compound contains 46.62 % by mass nitrogen. The molar mass of the compound is about 170 + 15 g/mole. a) Calculate the empirical formula of the compound. 6a) Ans: C2H8N3O b) What is the molecular formula of the compound? 6b) Ans: C4H16N6O2 7) 169 g FeCr2O4, 298 g ...
111 Exam I Outline
... Analysis of nitrogen showed that the compound contains 46.62 % by mass nitrogen. The molar mass of the compound is about 170 + 15 g/mole. a) Calculate the empirical formula of the compound. 6a) Ans: C2H8N3O b) What is the molecular formula of the compound? 6b) Ans: C4H16N6O2 7) 169 g FeCr2O4, 298 g ...
... Analysis of nitrogen showed that the compound contains 46.62 % by mass nitrogen. The molar mass of the compound is about 170 + 15 g/mole. a) Calculate the empirical formula of the compound. 6a) Ans: C2H8N3O b) What is the molecular formula of the compound? 6b) Ans: C4H16N6O2 7) 169 g FeCr2O4, 298 g ...
Minerals of Earth`s Crust Section 2 Physical
... • Describe seven physical properties that help distinguish one mineral from another. • List five special properties that may help identify certain minerals. ...
... • Describe seven physical properties that help distinguish one mineral from another. • List five special properties that may help identify certain minerals. ...
Compounds
... 40. Tin is too soft to be used by itself for practical purposes. It has been alloyed at different times with lead, antimony and copper to form metals collectively known as pewter. In all cases, it retains the crystalline structure of tin. What types of alloys are these? 41. Stainless steel is an exa ...
... 40. Tin is too soft to be used by itself for practical purposes. It has been alloyed at different times with lead, antimony and copper to form metals collectively known as pewter. In all cases, it retains the crystalline structure of tin. What types of alloys are these? 41. Stainless steel is an exa ...
transition metals
... • FAMILIES 3 TO 12 (TRANSITION METALS) – Do not fit into any other Family – Properties similar to one another but different from other Families – Good conductors of heat and electricity – Brightly colored (paints) – Most have 1 or 2 valence electrons – Lose either 1 (+1) or both (+2) BU ...
... • FAMILIES 3 TO 12 (TRANSITION METALS) – Do not fit into any other Family – Properties similar to one another but different from other Families – Good conductors of heat and electricity – Brightly colored (paints) – Most have 1 or 2 valence electrons – Lose either 1 (+1) or both (+2) BU ...
a-study-on-the
... acquired the structure of α glycine and it existed in zwitter ionic form, which resulted in the modifications of the properties. From the UV-Visible transmission spectrum, it was evident that the percentage of transmission (85.39%) was enhanced due to doping. This property can be used for optical ap ...
... acquired the structure of α glycine and it existed in zwitter ionic form, which resulted in the modifications of the properties. From the UV-Visible transmission spectrum, it was evident that the percentage of transmission (85.39%) was enhanced due to doping. This property can be used for optical ap ...
Chemistry Academic v. 2016
... Describe the evolution of atomic theory of the atom and how it contributed to the modern model of the atom. Differentiate between the mass number of an isotope and an average atomic mass of an element and distinguish among the isotopic forms of elements. Recognize discoveries from Daltons atomic the ...
... Describe the evolution of atomic theory of the atom and how it contributed to the modern model of the atom. Differentiate between the mass number of an isotope and an average atomic mass of an element and distinguish among the isotopic forms of elements. Recognize discoveries from Daltons atomic the ...
Crystals and photons*
... Academy and the opportunity they have afforded us of having our Annual Meeting in a truly academic atmosphere set amidst the beautiful scenery of Waltair, We appreciate very much the warmth of the welcome we have received. Our gathering here gives the Fellows of the Academy an opportunity of visitin ...
... Academy and the opportunity they have afforded us of having our Annual Meeting in a truly academic atmosphere set amidst the beautiful scenery of Waltair, We appreciate very much the warmth of the welcome we have received. Our gathering here gives the Fellows of the Academy an opportunity of visitin ...
CC 5 402-403..8121g ose chapter .. Page402
... whereas the flattened fourth ring is only linked through Hbonding to neighboring molecules. Unlike the crown-like conformation (C4v) and the recently observed boat-like ‘flattened cone’ conformation (C2v) in which the four intramolecular hydrogen bonds along the upper rim of CMCR are broken, the sco ...
... whereas the flattened fourth ring is only linked through Hbonding to neighboring molecules. Unlike the crown-like conformation (C4v) and the recently observed boat-like ‘flattened cone’ conformation (C2v) in which the four intramolecular hydrogen bonds along the upper rim of CMCR are broken, the sco ...