Coordination Compounds - Madison Public Schools
... Knowing the charge on a complex ion and the charge on each ligand, one can determine the oxidation number for the metal. Chemistry of Coordination Compounds ...
... Knowing the charge on a complex ion and the charge on each ligand, one can determine the oxidation number for the metal. Chemistry of Coordination Compounds ...
Chapter 24 Chemistry of Coordination Compounds
... Knowing the charge on a complex ion and the charge on each ligand, one can determine the oxidation number for the metal. Chemistry of Coordination Compounds ...
... Knowing the charge on a complex ion and the charge on each ligand, one can determine the oxidation number for the metal. Chemistry of Coordination Compounds ...
Co-ordination Chemistry with Macrocyclic Compounds
... ton exhibiting a hydrophobic behaviour. Their hydrophobic exteriors allow them to solubilize ionic substances in non-aqueous solvents and in membrane media. The most spectacular fact, at that time, was the dissolution of potassium permanganate in benzene by an 18crown-6, 5, reported by Pedersen [ 11 ...
... ton exhibiting a hydrophobic behaviour. Their hydrophobic exteriors allow them to solubilize ionic substances in non-aqueous solvents and in membrane media. The most spectacular fact, at that time, was the dissolution of potassium permanganate in benzene by an 18crown-6, 5, reported by Pedersen [ 11 ...
Lecture 26
... Stoichiometry of Complexes A species that bonds to a metal cation to form a complex is known as a ligand. The number of ligands is called the coordination number) The stabilization of a metal complex by a ligand with more than one donor atom is known as the chelate effect. ...
... Stoichiometry of Complexes A species that bonds to a metal cation to form a complex is known as a ligand. The number of ligands is called the coordination number) The stabilization of a metal complex by a ligand with more than one donor atom is known as the chelate effect. ...
{Ru(trpy)(bpy)} (trpy ) 2,2
... mediation can be attributed to the interaction mainly by the hole-transfer mechanism18 because DMcT2- itself is easily oxidized,10c,d showing that the highest occupied molecular orbital level possesses relatively high energy. The π system on DMcT2- is largely perturbed by thiol-thioamide tautomeriza ...
... mediation can be attributed to the interaction mainly by the hole-transfer mechanism18 because DMcT2- itself is easily oxidized,10c,d showing that the highest occupied molecular orbital level possesses relatively high energy. The π system on DMcT2- is largely perturbed by thiol-thioamide tautomeriza ...
full paper - Wayne State Chemistry Department
... partially filled d shells.[8,9] There has been one previous report of a (pyrazolato)vanadium complex, [(TpiPr2V(O)(µOH)(µ-PziPr2)V(O)(µ-OH)(µ-PziPr2)V(O)TpiPr2)], which was obtained by partial hydrolysis of a vanadium(IV) hydrotris(3,5-diisopropyl-1-pyrazolyl)borate complex.[16] The 3,5-diisopropylp ...
... partially filled d shells.[8,9] There has been one previous report of a (pyrazolato)vanadium complex, [(TpiPr2V(O)(µOH)(µ-PziPr2)V(O)(µ-OH)(µ-PziPr2)V(O)TpiPr2)], which was obtained by partial hydrolysis of a vanadium(IV) hydrotris(3,5-diisopropyl-1-pyrazolyl)borate complex.[16] The 3,5-diisopropylp ...
COLOURED IONS a solution of copper(II)sulphate is blue because
... Light of a certain wavelength is passed through a solution the greater the colour intensity, the greater the absorbance the concentration of each species in the complex is altered the mixture with the greatest absorbance identifies ratio of ligands and ions ...
... Light of a certain wavelength is passed through a solution the greater the colour intensity, the greater the absorbance the concentration of each species in the complex is altered the mixture with the greatest absorbance identifies ratio of ligands and ions ...
No Slide Title
... ight of a certain wavelength is passed through a solution the greater the colour intensity, the greater the absorbance the concentration of each species in the complex is altered the mixture with the greatest absorbance identifies ratio of ligands and ions ...
... ight of a certain wavelength is passed through a solution the greater the colour intensity, the greater the absorbance the concentration of each species in the complex is altered the mixture with the greatest absorbance identifies ratio of ligands and ions ...
Chem 4B First Midterm Review Sheet
... Ion exchange resins are insoluble materials that contain cations or anions that can be exchanged. The resins typically consist of a framework held together by strong chemical bonds. Positively or negatively charged functional groups are attached to this framework and each of these groups carries an ...
... Ion exchange resins are insoluble materials that contain cations or anions that can be exchanged. The resins typically consist of a framework held together by strong chemical bonds. Positively or negatively charged functional groups are attached to this framework and each of these groups carries an ...
Preparation of an Inorganic Complex Potassium
... between the central atom and the coordinating ligands varies with the nature of the species involved. They generally involve unshared electron pairs on the ligand. In some coordination compounds, the bonding may be described as largely covalent in character, whereas in others, electron sharing is re ...
... between the central atom and the coordinating ligands varies with the nature of the species involved. They generally involve unshared electron pairs on the ligand. In some coordination compounds, the bonding may be described as largely covalent in character, whereas in others, electron sharing is re ...
one of the tasks (coordination chemistry)
... link between the example and the principle may be obvious to the instructors, students do not necessarily understand the connection. Students may understand examples of a principle when the examples have been presented to them but their understanding is challenged more deeply if they are required to ...
... link between the example and the principle may be obvious to the instructors, students do not necessarily understand the connection. Students may understand examples of a principle when the examples have been presented to them but their understanding is challenged more deeply if they are required to ...
Exam 3 Review Key
... b) Water is a pi-base, since it uses one lone pair to sigma bond to the metal, and the leftover lone pair can be donated to the metal in a pi fashion. Ammonia is not a pi-base since it has no more lone pairs after donating one in a sigma bond. Pi-bases increase the energy of the t2g orbitals and dec ...
... b) Water is a pi-base, since it uses one lone pair to sigma bond to the metal, and the leftover lone pair can be donated to the metal in a pi fashion. Ammonia is not a pi-base since it has no more lone pairs after donating one in a sigma bond. Pi-bases increase the energy of the t2g orbitals and dec ...
Lecture 5 - Stereochemistry in Transition Metal
... born it was a very big shock. In fact for the first three days I didn't really want to know," my mother admitted. In the late 1950s and 1960s pregnant women across the globe reached for a recommended remedy for bouts of morning sickness - thalidomide. Around 10,000 babies were born with disabilities ...
... born it was a very big shock. In fact for the first three days I didn't really want to know," my mother admitted. In the late 1950s and 1960s pregnant women across the globe reached for a recommended remedy for bouts of morning sickness - thalidomide. Around 10,000 babies were born with disabilities ...
( i ) in enantioselective nhk reaction
... asymmetric reactions and catalyst concentration of 0.025 M gave the best results In contrast to salen ligand 1, ligand 2 was able to effect an enantioselective addition of allyl iodide ...
... asymmetric reactions and catalyst concentration of 0.025 M gave the best results In contrast to salen ligand 1, ligand 2 was able to effect an enantioselective addition of allyl iodide ...
Experiment 8 Chelatometric Analysis of the Complex for Cobalt
... The murexide anion forms a complex with cobalt(II) ions, bonding to the metal by means of the central nitrogen and the two carbonyl groups (indicated by asterisks). At a pH of six or less, the CoH4 D+ complex is orange, whereas in alkaline solution the metal-murexide complex is yellow. Moreover, the ...
... The murexide anion forms a complex with cobalt(II) ions, bonding to the metal by means of the central nitrogen and the two carbonyl groups (indicated by asterisks). At a pH of six or less, the CoH4 D+ complex is orange, whereas in alkaline solution the metal-murexide complex is yellow. Moreover, the ...
Series S Sensor Chip NTA - GE Healthcare Life Sciences
... • Prepare the histidine-tagged ligand in running buffer. Concentrations below 0.2 μM (30 μg/mL for a protein of molecular weight 150 000) are normally sufficient. If the capture level is too high there is a risk that the ligand may dissociate too fast during the analysis cycle. • Inject ligand solut ...
... • Prepare the histidine-tagged ligand in running buffer. Concentrations below 0.2 μM (30 μg/mL for a protein of molecular weight 150 000) are normally sufficient. If the capture level is too high there is a risk that the ligand may dissociate too fast during the analysis cycle. • Inject ligand solut ...
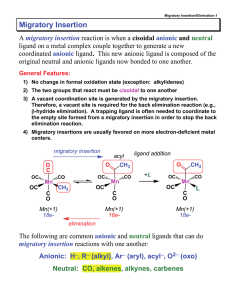
Migratory Insertion - vrg
... coordinated anionic ligand. This new anionic ligand is composed of the original neutral and anionic ligands now bonded to one another. General Features: 1) No change in formal oxidation state (exception: alkylidenes) 2) The two groups that react must be cisoidal to one another 3) A vacant coordinati ...
... coordinated anionic ligand. This new anionic ligand is composed of the original neutral and anionic ligands now bonded to one another. General Features: 1) No change in formal oxidation state (exception: alkylidenes) 2) The two groups that react must be cisoidal to one another 3) A vacant coordinati ...
d-sub shells. The first row runs from scandium to zinc
... ight of a certain wavelength is passed through a solution the greater the colour intensity, the greater the absorbance the concentration of each species in the complex is altered the mixture with the greatest absorbance identifies ratio of ligands and ions ...
... ight of a certain wavelength is passed through a solution the greater the colour intensity, the greater the absorbance the concentration of each species in the complex is altered the mixture with the greatest absorbance identifies ratio of ligands and ions ...
No Slide Title
... ight of a certain wavelength is passed through a solution the greater the colour intensity, the greater the absorbance the concentration of each species in the complex is altered the mixture with the greatest absorbance identifies ratio of ligands and ions ...
... ight of a certain wavelength is passed through a solution the greater the colour intensity, the greater the absorbance the concentration of each species in the complex is altered the mixture with the greatest absorbance identifies ratio of ligands and ions ...
Synthesis of chiral furanoside diphosphinite and thioether-phosphinite compounds derived from
... form the linear and branched alkyl toward the β-hydride elimination is a key step in the determination of the regioselectivity. There is then the migratory insertion of the alkyl group to one of the coordinated carbon monoxides, the oxidative addition of molecular hydrogen, the reductive elimination ...
... form the linear and branched alkyl toward the β-hydride elimination is a key step in the determination of the regioselectivity. There is then the migratory insertion of the alkyl group to one of the coordinated carbon monoxides, the oxidative addition of molecular hydrogen, the reductive elimination ...
Electrolytes 1. List whether each of the following is a strong, weak, or
... oxygen carrier being related to the ability of the iron atoms to coordinate oxygen molecules reversibly. Other biologically important coordination compounds include chlorophyll (a magnesiumporphyrin complex) and vitamin B12, a complex of cobalt with a macrocyclic ligand known as corrin. Coordination ...
... oxygen carrier being related to the ability of the iron atoms to coordinate oxygen molecules reversibly. Other biologically important coordination compounds include chlorophyll (a magnesiumporphyrin complex) and vitamin B12, a complex of cobalt with a macrocyclic ligand known as corrin. Coordination ...
Document
... include the hydroxide ion (OH–), water molecule (H2O), ammonia molecule (NH3), cyanide ion (CN–) and halide ions (F–, Cl–, Br– and I–). ...
... include the hydroxide ion (OH–), water molecule (H2O), ammonia molecule (NH3), cyanide ion (CN–) and halide ions (F–, Cl–, Br– and I–). ...
E16 THE COPPER(II) SULFATE/AMMONIA COMPLEX A complex
... include the hydroxide ion (OH–), water molecule (H2O), ammonia molecule (NH3), cyanide ion (CN–) and halide ions (F–, Cl–, Br– and I–). ...
... include the hydroxide ion (OH–), water molecule (H2O), ammonia molecule (NH3), cyanide ion (CN–) and halide ions (F–, Cl–, Br– and I–). ...
Ligand
4-3D-balls.png?width=300)
In coordination chemistry, a ligand (/lɪɡənd/) is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from covalent to ionic. Furthermore, the metal-ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic ""ligand.""Metals and metalloids are bound to ligands in virtually all circumstances, although gaseous ""naked"" metal ions can be generated in high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection is a critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemistry.Ligands are classified in many ways like : their charge, their size (bulk), the identity of the coordinating atom(s), and the number of electrons donated to the metal (denticity or hapticity). The size of a ligand is indicated by its cone angle.