UV-Visible Spectra of Aquavanadium Complexes
... It is not always the case that TD-DFT calculations agree with experiment, and there is every reason to be critical about the calculational results. Very carefully compare the TD-DFT calculations with experiment. In many cases, artifacts will arise: charge transfer states tend to appear much too low ...
... It is not always the case that TD-DFT calculations agree with experiment, and there is every reason to be critical about the calculational results. Very carefully compare the TD-DFT calculations with experiment. In many cases, artifacts will arise: charge transfer states tend to appear much too low ...
NAME: Chem 1b, 2005, 3rd
... a) What is the arrangement of the anion anions in a crystal structure of spinel? Cloest packing of oxide anions is expected. Since the crystal has octahedral (cubic) symmetry, expect cubic closest packing (ccp). b) The magnesium cations are found in tetrahedral holes. What fraction of the tetrahedra ...
... a) What is the arrangement of the anion anions in a crystal structure of spinel? Cloest packing of oxide anions is expected. Since the crystal has octahedral (cubic) symmetry, expect cubic closest packing (ccp). b) The magnesium cations are found in tetrahedral holes. What fraction of the tetrahedra ...
Electronic Spectroscopy: Interpretation
... transitions. We can see this in the spectra as the 0-2 has the greatest absorbance. If the potential energy wells of the ground and excited states were perfectly eclipsed on top of one another, then the 0-0 transition would be the most probable transition with the highest intensity band. This howeve ...
... transitions. We can see this in the spectra as the 0-2 has the greatest absorbance. If the potential energy wells of the ground and excited states were perfectly eclipsed on top of one another, then the 0-0 transition would be the most probable transition with the highest intensity band. This howeve ...
5.04 Principles of Inorganic Chemistry II
... Lecture 10: General Electronic Considerations of Metal-Ligand Complexes Metal complexes are Lewis acid-base adducts formed between metal ions (the acid) and ligands (the base). ...
... Lecture 10: General Electronic Considerations of Metal-Ligand Complexes Metal complexes are Lewis acid-base adducts formed between metal ions (the acid) and ligands (the base). ...
Homework 1 - IONiC / VIPEr
... There are 3 ways that inorganic chemists often classify complexes: metal oxidation state, dn count, and total valence electron count. While a useful formalism, you should be aware that an isolated Cr+6 ion is really not present in a complex ion. For this reason, metal ions are usually written with r ...
... There are 3 ways that inorganic chemists often classify complexes: metal oxidation state, dn count, and total valence electron count. While a useful formalism, you should be aware that an isolated Cr+6 ion is really not present in a complex ion. For this reason, metal ions are usually written with r ...
Questions and answers Coordination
... Ans. (a) [Cr(ox)3]3– It is definitely chiral; two chelating ligands on an octahedral framework are sufficient do impart chirality to the complex. The two enantiomers provide the left and right of a plane of reflection. (b) cis‐[PtCl2(en)] This molecule would be chiral be ...
... Ans. (a) [Cr(ox)3]3– It is definitely chiral; two chelating ligands on an octahedral framework are sufficient do impart chirality to the complex. The two enantiomers provide the left and right of a plane of reflection. (b) cis‐[PtCl2(en)] This molecule would be chiral be ...
Nomenclature for d-block complexes Writing chemical names
... (also known as, “how to name compounds and write formulae”) ...
... (also known as, “how to name compounds and write formulae”) ...
Get Day 18 - Mattson Creighton
... Scenario #2. Strong field ligands. When Δo is larger as is the case with _______ field ligands, the eg orbitals are generally too high in energy (too antibonding). Populating them is not done very often, although examples exist. As usual, the ligands deliver 12 electrons. This limits the metal catio ...
... Scenario #2. Strong field ligands. When Δo is larger as is the case with _______ field ligands, the eg orbitals are generally too high in energy (too antibonding). Populating them is not done very often, although examples exist. As usual, the ligands deliver 12 electrons. This limits the metal catio ...
Assignment 8 Solutions
... 11.11 a. d 8 (Oh) is t2g6eg2. There is only one configuration for this, so it is 3A1g. b. d 5 high spin is t2g3eg2, for which there is only one configuration, so it is 6A1g. d 5 low spin is t2g5eg0. The unpaired electron can be in any of the three t2g orbitals, making this a triply degenerate T term ...
... 11.11 a. d 8 (Oh) is t2g6eg2. There is only one configuration for this, so it is 3A1g. b. d 5 high spin is t2g3eg2, for which there is only one configuration, so it is 6A1g. d 5 low spin is t2g5eg0. The unpaired electron can be in any of the three t2g orbitals, making this a triply degenerate T term ...
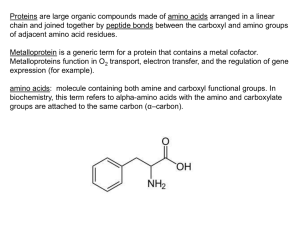
amino acids
... 2. The biosynthesis of critical signaling molecules used for control of development and homeostasis. ...
... 2. The biosynthesis of critical signaling molecules used for control of development and homeostasis. ...
Coordination Chemistry II: Bonding
... Theories of Electronic Structure • Valence Bond Theory – Not commonly used, but the hybrid notation is still common. • Crystal Field Theory – An electrostatic approach used to describe the splitting in metal d-orbital energies. Does not describe bonding. • Ligand Field Theory – A more complete desc ...
... Theories of Electronic Structure • Valence Bond Theory – Not commonly used, but the hybrid notation is still common. • Crystal Field Theory – An electrostatic approach used to describe the splitting in metal d-orbital energies. Does not describe bonding. • Ligand Field Theory – A more complete desc ...
Lecture 17.The d-Block Elements.General properties
... a) electronic transitions of d-electrons within the d subshell. These are known as d→d transitions. d0 and d10 metal ions do not show these transitions. b) electronic transitions from the metal ion to the ligand (M→L transitions) or ligand to the metal ion (L→M transitions), which are known as charg ...
... a) electronic transitions of d-electrons within the d subshell. These are known as d→d transitions. d0 and d10 metal ions do not show these transitions. b) electronic transitions from the metal ion to the ligand (M→L transitions) or ligand to the metal ion (L→M transitions), which are known as charg ...
Spin crossover

Spin Crossover (SCO), sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a magnetic field.With regard to a ligand field and ligand field theory, the change in spin state is a transition from a low spin (LS) ground state electron configuration to a high spin (HS) ground state electron configuration of the metal’s d atomic orbitals (AOs), or vice versa. The magnitude of the ligand field splitting along with the pairing energy of the complex determines whether it will have a LS or HS electron configuration. A LS state occurs because the ligand field splitting (Δ) is greater than the pairing energy of the complex (which is an unfavorable process).Figure 1 is a simplified illustration of the metal’s d orbital splitting in the presence of an octahedral ligand field. A large splitting between the t2g and eg AOs requires a substantial amount of energy for the electrons to overcome the energy gap (Δ) to comply with Hund’s Rule. Therefore, electrons will fill the lower energy t2g orbitals completely before populating the higher energy eg orbitals. Conversely, a HS state occurs with weaker ligand fields and smaller orbital splitting. In this case the energy required to populate the higher levels is substantially less than the pairing energy and the electrons fill the orbitals according to Hund’s Rule by populating the higher energy orbitals before pairing with electrons in the lower lying orbitals. An example of a metal ion that can exist in either a LS or HS state is Fe3+ in an octahedral ligand field. Depending on the ligands that are coordinated to this complex the Fe3+ can attain a LS or a HS state, as in Figure 1.Spin crossover refers to the transitions between high to low, or low to high, spin states. This phenomenon is commonly observed with some first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves are a common representation of SCO phenomenon with the most commonly observed types depicted in Figure 2 in which γHS (the high-spin molar fraction) is plotted vs. T. The figure shows a gradual spin transition (left), an abrupt transition with hysteresis (middle) and a two-step transition (right). For a transition to be considered gradual, it typically takes place over a large temperature range, even up to several hundred K, whereas for a transition to be considered abrupt, it should take place within 10 K or less.These curves indicate that a spin transition has occurred in a metal complex as temperature changed. The gradual transition curve is an indication that not all metal centers within the complex are undergoing the transition at the same temperature. The abrupt spin change with hysteresis indicates a strong cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of external stimuli (temperature in this case) for the two phenomena, namely LS → HS and HS → LS. The two-step transition is relatively rare but is observed, for example, with dinuclear SCO complexes for which the spin transition in one metal center renders the transition in the second metal center less favorable.There are several types of spin crossover that can occur in a complex; some of them are light induced excited state spin trapping (LIESST), ligand-driven light induced spin change (LD-LISC), and charge transfer induced spin transition (CTIST).