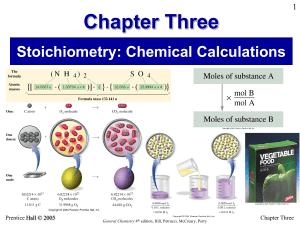
Chapter Three - CNG Chemistry
... quantity. Doing this ensures that the precision of the calculated results is limited only by the least precisely known quantity. In part (d), this simple fact should deter you from mistakenly multiplying instead of dividing by Avogadro’s number: Individual atoms are exceedingly small and possess mas ...
... quantity. Doing this ensures that the precision of the calculated results is limited only by the least precisely known quantity. In part (d), this simple fact should deter you from mistakenly multiplying instead of dividing by Avogadro’s number: Individual atoms are exceedingly small and possess mas ...
Higher Chemistry Resources Guide - Glow Blogs
... demonstrating the formation of the hydrogen bonded lattice structure which causes the anomalously large volume for frozen water. Hydrogen bonding is also responsible for the surface tension of water can be demonstrated using classic experiments such as the floating needle on the surface of a glass o ...
... demonstrating the formation of the hydrogen bonded lattice structure which causes the anomalously large volume for frozen water. Hydrogen bonding is also responsible for the surface tension of water can be demonstrated using classic experiments such as the floating needle on the surface of a glass o ...
exam2gc1sum11+key
... B. 0.895 J/g·oC C. 355 J/g·oC D. 1.49 J/g·oC ____ 17. The thermochemical equation for the combustion of styrene, C8H8, under 1 atm pressure and 25°C is the following. Find the standard enthalpy of formation of styrene at 25°C. (Given: ΔH°f[CO2(g)] = – 394 kJ/mol, ΔH°f[H2O(l)] = –286 kJ/mol) C8H8 + 1 ...
... B. 0.895 J/g·oC C. 355 J/g·oC D. 1.49 J/g·oC ____ 17. The thermochemical equation for the combustion of styrene, C8H8, under 1 atm pressure and 25°C is the following. Find the standard enthalpy of formation of styrene at 25°C. (Given: ΔH°f[CO2(g)] = – 394 kJ/mol, ΔH°f[H2O(l)] = –286 kJ/mol) C8H8 + 1 ...
File
... gained or shared when it reacts and forms chemical bonds. The change in oxidation state of a species tells you if it has undergone oxidation or reduction. The usual Oxidation Numbers for common elements in their compounds ELEMENT H, Na, K Mg, Ca F, Cl, Br, I O ...
... gained or shared when it reacts and forms chemical bonds. The change in oxidation state of a species tells you if it has undergone oxidation or reduction. The usual Oxidation Numbers for common elements in their compounds ELEMENT H, Na, K Mg, Ca F, Cl, Br, I O ...
Manual Physical Chemistry III
... The surface tension of liquids measured with the stalagmometer Surface tension of liquids The molecules of liquids attract each other by cohesive forces resulting into small distances between the molecules (on the order of 0.1 nm). Thus the compressibility of liquids is lower than that of gas, while ...
... The surface tension of liquids measured with the stalagmometer Surface tension of liquids The molecules of liquids attract each other by cohesive forces resulting into small distances between the molecules (on the order of 0.1 nm). Thus the compressibility of liquids is lower than that of gas, while ...
Acids and Bases
... Suggested that acids contain one or more hydrogen atoms which can be replaced by metal atoms to produce salts. e.g. HSCN is an acid because the H atom can be replace by a metal to form a salt, such as NaSCN. 1884 – Svante Arrhenius acids and bases: ...
... Suggested that acids contain one or more hydrogen atoms which can be replaced by metal atoms to produce salts. e.g. HSCN is an acid because the H atom can be replace by a metal to form a salt, such as NaSCN. 1884 – Svante Arrhenius acids and bases: ...
Acid‒base reaction
... Lewis definition The hydrogen requirement of Arrhenius and Brønsted–Lowry was removed by the Lewis definition of acid–base reactions, devised by Gilbert N. Lewis in 1923,[12] in the same year as Brønsted–Lowry, but it was not elaborated by him until 1938.[2] Instead of defining acid–base reactions i ...
... Lewis definition The hydrogen requirement of Arrhenius and Brønsted–Lowry was removed by the Lewis definition of acid–base reactions, devised by Gilbert N. Lewis in 1923,[12] in the same year as Brønsted–Lowry, but it was not elaborated by him until 1938.[2] Instead of defining acid–base reactions i ...
QUESTION BANK CHEMISTRY-XII THE SOLID STATE CHAPTER
... structure? 2. Name an element with which silicon can be doped to give a p-type semiconductor. 3. A cubic solid is made of two elements X and Y. Atoms Y are at the corners of the cube and X at the body centre. What is the formula of the compound? 4. Why is common salt sometimes yellow instead of bein ...
... structure? 2. Name an element with which silicon can be doped to give a p-type semiconductor. 3. A cubic solid is made of two elements X and Y. Atoms Y are at the corners of the cube and X at the body centre. What is the formula of the compound? 4. Why is common salt sometimes yellow instead of bein ...
Higher Chemistry Resources Guide - Glow Blogs
... or lab floor and experiment with different arrangements. The story of the development of the modern Periodic Table could be explored. Elements can be extracted from their compounds: Silicon can be extracted from sand using magnesium. (See Classic Chemistry Demonstrations, Lister, T., The Royal Socie ...
... or lab floor and experiment with different arrangements. The story of the development of the modern Periodic Table could be explored. Elements can be extracted from their compounds: Silicon can be extracted from sand using magnesium. (See Classic Chemistry Demonstrations, Lister, T., The Royal Socie ...
Section 1 Describing Chemical Reactions Chapter 8
... balancing equations by inspection, continued 3. Balance the formula equation according to the law of conservation of mass. • Balance the different types of atoms one at a time. • First balance the atoms of elements that are combined and that appear only once on each side of the equation. • Balance p ...
... balancing equations by inspection, continued 3. Balance the formula equation according to the law of conservation of mass. • Balance the different types of atoms one at a time. • First balance the atoms of elements that are combined and that appear only once on each side of the equation. • Balance p ...
a) How many moles of water are created when 108 moles of oxygen
... 3. Balance the following equations by filling in the blanks with the correct coefficient. Use the table below each reaction to show your work. It is usually easiest to do the element found in the most compounds LAST and to start with the element that is simplest to balance. An example is provided fo ...
... 3. Balance the following equations by filling in the blanks with the correct coefficient. Use the table below each reaction to show your work. It is usually easiest to do the element found in the most compounds LAST and to start with the element that is simplest to balance. An example is provided fo ...
Grossmont College Chemistry 120 Laboratory Manual 6th Edition
... Prepare your Laboratory Report on each experiment with care. If you prepare your own report form, use a permanently bound notebook as prescribed by your instructor, preferably one with 5-mm cross rulings. Always have the data entry portion prepared in advance, and record data directly in your final ...
... Prepare your Laboratory Report on each experiment with care. If you prepare your own report form, use a permanently bound notebook as prescribed by your instructor, preferably one with 5-mm cross rulings. Always have the data entry portion prepared in advance, and record data directly in your final ...
Redox Reactions
... Electron Transfer Reactions • oxidation-reduction or redox reactions are Electron transfer reactions. • Redox reactions can result in the ...
... Electron Transfer Reactions • oxidation-reduction or redox reactions are Electron transfer reactions. • Redox reactions can result in the ...
ism ismismismismismrapidrevisionquestionsismismismismismism
... (i) Combination between nitrogen and hydrogen to form ammonia in the presence of iron catalyst in Haber’s process. Fe N2 + 3H2 2NH3 (ii) Zeolites catalyst ZSM-5 is used to convert alcohol to gasoline by dehydration. (iii) The enzyme zymase converts glucose into ethyl alcohol and carbon dioxide. Zyma ...
... (i) Combination between nitrogen and hydrogen to form ammonia in the presence of iron catalyst in Haber’s process. Fe N2 + 3H2 2NH3 (ii) Zeolites catalyst ZSM-5 is used to convert alcohol to gasoline by dehydration. (iii) The enzyme zymase converts glucose into ethyl alcohol and carbon dioxide. Zyma ...
380 KB / 39 pages
... above the surface and spilling out of the cylinder and falling toward the ground as the fog is carried down by the cold dense CO2(g) (and the cooled air) spilling out of the cylinder as well. Some of the CO2(g) dissolves in the solution forming some carbonic acid, (HO)2CO(aq), which can react with t ...
... above the surface and spilling out of the cylinder and falling toward the ground as the fog is carried down by the cold dense CO2(g) (and the cooled air) spilling out of the cylinder as well. Some of the CO2(g) dissolves in the solution forming some carbonic acid, (HO)2CO(aq), which can react with t ...
1b-Redox FIB notes and practice
... 2. The oxidation number for metals in an ionic compound is just their ionic charge. Ie. The oxidation number of Ca in CaBr2 is _____, the oxidation number for Fe in Fe2O3 is _______. Ex) 2NaBr(aq) + Hg(NO3)2(aq) --> 2NaNO3(aq) + HgBr2(s) 3. The oxidation number of hydrogen in most of its compound is ...
... 2. The oxidation number for metals in an ionic compound is just their ionic charge. Ie. The oxidation number of Ca in CaBr2 is _____, the oxidation number for Fe in Fe2O3 is _______. Ex) 2NaBr(aq) + Hg(NO3)2(aq) --> 2NaNO3(aq) + HgBr2(s) 3. The oxidation number of hydrogen in most of its compound is ...
Some basic concepts of chemistry
... Elements are pure substances that cannot be decomposed into simpler substances by chemical changes. It consists of only one type of atoms. e.g. sodium (Na), silver (Ag), oxygen (O2 ), Chlorine (Cl 2 ) etc. Presently 118 different elements are known, out of which 92 are naturally occurring. Compounds ...
... Elements are pure substances that cannot be decomposed into simpler substances by chemical changes. It consists of only one type of atoms. e.g. sodium (Na), silver (Ag), oxygen (O2 ), Chlorine (Cl 2 ) etc. Presently 118 different elements are known, out of which 92 are naturally occurring. Compounds ...
Lecture 2
... In most acid-base reactions HOMO-LUMO combinations lead to new HOMO-LUMO of the product ...
... In most acid-base reactions HOMO-LUMO combinations lead to new HOMO-LUMO of the product ...
Inorganic chemistry

Inorganic chemistry deals with the synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds), which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, and there is much overlap, most importantly in the sub-discipline of organometallic chemistry. It has applications in every aspect of the chemical industry–including catalysis, materials science, pigments, surfactants, coatings, medicine, fuel, and agriculture.