
Chapter 4: Chemical Reactions Elements can be characterized as
... Table 4-11 (List of common cations and anions) Binary molecular compounds (Mostly two nonmetals bonded together) Use Greek and Latin prefixes instead of Roman numerals and suffixes. Examples: SO2 – sulfur dioxide; SO3 – sulfur trioxide; As4O6 – tetraarsenic hexoxide Learn the common prefixes (pg. 14 ...
... Table 4-11 (List of common cations and anions) Binary molecular compounds (Mostly two nonmetals bonded together) Use Greek and Latin prefixes instead of Roman numerals and suffixes. Examples: SO2 – sulfur dioxide; SO3 – sulfur trioxide; As4O6 – tetraarsenic hexoxide Learn the common prefixes (pg. 14 ...
Answers to 2017 Chemistry Exam Review Compounds and
... have negative values for things like volume and b/c the values are proportional to the lowest temperature being absolute zero (when there is no molecular motion). 80. When temperature rises, volume rises in a balloon or pressure rises in a rigid container. This is b/c the gas molecules move faster a ...
... have negative values for things like volume and b/c the values are proportional to the lowest temperature being absolute zero (when there is no molecular motion). 80. When temperature rises, volume rises in a balloon or pressure rises in a rigid container. This is b/c the gas molecules move faster a ...
File - Mr. Walsh`s AP Chemistry
... named by describing the molecular formula, using prefixes for the numbers. o You will need to memorize the number prefixes for the numbers 1–10. o E.g., P2O5 is diphosphorus pentoxide. **Note that the prefix “mono—“ is never used with the first element. SO3 is simply sulfur trioxide. However, “mono— ...
... named by describing the molecular formula, using prefixes for the numbers. o You will need to memorize the number prefixes for the numbers 1–10. o E.g., P2O5 is diphosphorus pentoxide. **Note that the prefix “mono—“ is never used with the first element. SO3 is simply sulfur trioxide. However, “mono— ...
Chapter 2 - Chemistry
... Periodic Table of the Elements Dmitri Mendeleev and J. Lothar Meyer - tabular arrangement of elements in rows and columns, highlighting the regular repetition of properties of the elements - today arrangement of elements by atomic number (represents element by symbol, atomic number and atomic mass) ...
... Periodic Table of the Elements Dmitri Mendeleev and J. Lothar Meyer - tabular arrangement of elements in rows and columns, highlighting the regular repetition of properties of the elements - today arrangement of elements by atomic number (represents element by symbol, atomic number and atomic mass) ...
CHEM 11 Practice Exam 2
... CHEM 11 Practice Exam 2 1) What is the term for the electrons in the highest s and p sublevels in an atom that can undergo reaction and form chemical bonds? A) s electrons B) p electrons C) bonding electrons D) valence electrons E) none of the above 2) Which element has the following electron config ...
... CHEM 11 Practice Exam 2 1) What is the term for the electrons in the highest s and p sublevels in an atom that can undergo reaction and form chemical bonds? A) s electrons B) p electrons C) bonding electrons D) valence electrons E) none of the above 2) Which element has the following electron config ...
Bonding in Atoms
... • Attraction of free floating electrons in the cloud • Flow of electrons allows for great conductivity • Also allows for malleability • Often arranged in a crystalline structure • Alloys is a mixture that includes at least one metal and enhances the properties of the metal ...
... • Attraction of free floating electrons in the cloud • Flow of electrons allows for great conductivity • Also allows for malleability • Often arranged in a crystalline structure • Alloys is a mixture that includes at least one metal and enhances the properties of the metal ...
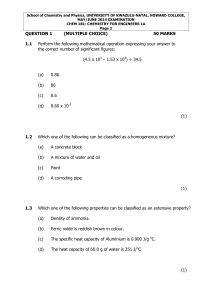
School of Chemistry
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
Chapter 1: The Nature of Analytical Chemistry
... elements and compounds in a sample. – 2. Quantitative Analysis – determines identity of species (if unknown) and the amount of each substance in a sample. ...
... elements and compounds in a sample. – 2. Quantitative Analysis – determines identity of species (if unknown) and the amount of each substance in a sample. ...
Classification of Matter
... Components are evenly mixed. (More pure than heterogeneous) - Cannot see the parts. Salt water contains salt and water, but are mixed all the way to the atomic level , but it can still be separated by physical means. Seawater distillation plant ...
... Components are evenly mixed. (More pure than heterogeneous) - Cannot see the parts. Salt water contains salt and water, but are mixed all the way to the atomic level , but it can still be separated by physical means. Seawater distillation plant ...
final exam review packet
... Be able to tell the difference between chemical and physical changes (definition, examples) Know what different separation techniques can separate (elements/compound/mix) Know the law of conservation of matter EOC Targets: C-Matter-101. I can define what matter is. C-Matter-102. I can differentiate ...
... Be able to tell the difference between chemical and physical changes (definition, examples) Know what different separation techniques can separate (elements/compound/mix) Know the law of conservation of matter EOC Targets: C-Matter-101. I can define what matter is. C-Matter-102. I can differentiate ...
V. Chemical reactions
... Law of Conservation of Mass a. In a chemical reaction mass is neither lost nor gained. i. Atoms are rearranged b. Equations are balanced to show Conservation of Mass ...
... Law of Conservation of Mass a. In a chemical reaction mass is neither lost nor gained. i. Atoms are rearranged b. Equations are balanced to show Conservation of Mass ...
1. What are micelles? Give two examples of micellar systems. Sol. A
... Electron donating groups are needed as the R groups because these can stabilize the propagating species by resonance. Examples: ...
... Electron donating groups are needed as the R groups because these can stabilize the propagating species by resonance. Examples: ...
Eperimental studies of V.Ostwald and J.van Hoff
... process (patent 1902), used in the manufacture of nitric acid, although the basic chemistry had been patented some 64 years earlier by Kuhlmann, when it was probably of only academic interest due to the lack of a significant source of ammonia. That may have still been the state of affairs in 1902, a ...
... process (patent 1902), used in the manufacture of nitric acid, although the basic chemistry had been patented some 64 years earlier by Kuhlmann, when it was probably of only academic interest due to the lack of a significant source of ammonia. That may have still been the state of affairs in 1902, a ...
The structure of Matter
... O Compounds that contain only carbon and hydrogen are called hydrocarbons. O Two of the simplest hydrocarbons are methane and ethane. O Many hydrocarbons are used as fuels. ...
... O Compounds that contain only carbon and hydrogen are called hydrocarbons. O Two of the simplest hydrocarbons are methane and ethane. O Many hydrocarbons are used as fuels. ...
Review Worksheet
... c) These particles are considered to be dimensionless points which occupy zero volume. The volume of real gas molecules is assumed to be __________ for most purposes. This above statement is NOT TRUE. Real gas molecules do occupy volume and it does have an impact on the behavior of the gas. This imp ...
... c) These particles are considered to be dimensionless points which occupy zero volume. The volume of real gas molecules is assumed to be __________ for most purposes. This above statement is NOT TRUE. Real gas molecules do occupy volume and it does have an impact on the behavior of the gas. This imp ...
PUC Schools - cloudfront.net
... Standard 7: Chemical Thermodynamics 27. (7.c) When water changes phase from liquid to gas, the process is a) Exothermic b) Endothermic c) Neutral d) Kinetic 28. (7.c) The temperature of iced water melting is _____ oC. The temperature of boiling water is _____ oC. a) 100, 200 b) 0, 100 c) 100, 0 d) ...
... Standard 7: Chemical Thermodynamics 27. (7.c) When water changes phase from liquid to gas, the process is a) Exothermic b) Endothermic c) Neutral d) Kinetic 28. (7.c) The temperature of iced water melting is _____ oC. The temperature of boiling water is _____ oC. a) 100, 200 b) 0, 100 c) 100, 0 d) ...
Writing Chemical Formulas and Chemical Reactions
... Balancing Equations All chemical equations must be balanced so that they are consistent with the Law of Conservation of Mass. Here are some suggestions for balancing equations: 1. When balancing equations, always start with the “ugliest” molecule first (polyatomics). 2. To balance, place the desired ...
... Balancing Equations All chemical equations must be balanced so that they are consistent with the Law of Conservation of Mass. Here are some suggestions for balancing equations: 1. When balancing equations, always start with the “ugliest” molecule first (polyatomics). 2. To balance, place the desired ...
Review 2 key - Home [www.petoskeyschools.org]
... Fission- splitting of a larger nucleus into 2 or smaller nuclei. Releases energy. Fusion releases more energy than fission. 20 In Rutheford’s gold foil experiment, what 3 possible things happened to the particles when fired at the gold foil? What conclusions can be determined from this? Particles c ...
... Fission- splitting of a larger nucleus into 2 or smaller nuclei. Releases energy. Fusion releases more energy than fission. 20 In Rutheford’s gold foil experiment, what 3 possible things happened to the particles when fired at the gold foil? What conclusions can be determined from this? Particles c ...
(1) Dissolves, accompanied by evolution of flammable gas (2
... SELECT TWO OF THE FOUR ESSAY QUESTIONS, NUMBERED 6 THROUGH 9. (Additional essays will not be scored.) ...
... SELECT TWO OF THE FOUR ESSAY QUESTIONS, NUMBERED 6 THROUGH 9. (Additional essays will not be scored.) ...
Ductility-the ability to be stretched into wires
... Does the paper change its chemistry (chemical identity) and form a new substance with different properties? Is the ability to be torn a physical or chemical property? Physical Property: Property that can be tested/observed without changing chemical identity of the substance; can be undone ...
... Does the paper change its chemistry (chemical identity) and form a new substance with different properties? Is the ability to be torn a physical or chemical property? Physical Property: Property that can be tested/observed without changing chemical identity of the substance; can be undone ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.



















![Review 2 key - Home [www.petoskeyschools.org]](http://s1.studyres.com/store/data/000860497_1-e3bea510ba504d09bc42d6f5e4936390-300x300.png)


