Enthalpy, Entropy, Mollier Diagram and Steam
... ∆sY = (+3,000 kJ/kg)/(280 °K) = + 10.71 kJ/kg.°K {Due to heat gain by Planet Y} c) Can convectional heat transfer occur between any of two planets in this solar system? Solution/Answer: Convectional heat transfer is dependent on bulk movement of a fluid (gaseous or liquid) and, therefore, it can onl ...
... ∆sY = (+3,000 kJ/kg)/(280 °K) = + 10.71 kJ/kg.°K {Due to heat gain by Planet Y} c) Can convectional heat transfer occur between any of two planets in this solar system? Solution/Answer: Convectional heat transfer is dependent on bulk movement of a fluid (gaseous or liquid) and, therefore, it can onl ...
Boundless Study Slides
... • internal energy The sum of all energy present in the system, including kinetic and potential energy; equivalently, the energy needed to create a system, excluding the energy necessary to displace its surroundings. • internal energy The sum of all energy present in the system, including kinetic and ...
... • internal energy The sum of all energy present in the system, including kinetic and potential energy; equivalently, the energy needed to create a system, excluding the energy necessary to displace its surroundings. • internal energy The sum of all energy present in the system, including kinetic and ...
The Second Law - chem.uwec.edu
... see why, consider the contraction and expansion of a gas. When a gas contracts isothermally, the kinetic energy of the atoms becomes localized. When it expands, the locations of the particles become more widely dispersed and so too does their kinetic energy. Although it is easy to relate the spontan ...
... see why, consider the contraction and expansion of a gas. When a gas contracts isothermally, the kinetic energy of the atoms becomes localized. When it expands, the locations of the particles become more widely dispersed and so too does their kinetic energy. Although it is easy to relate the spontan ...
A Heat Transfer Textbook by John H. Lienhard IV and John H
... are in. If you leave the room, some small buoyancy-driven (or convective) motion of the air will continue because the walls can never be perfectly isothermal. Such processes go on in all plant and animal life and in the air around us. They occur throughout the earth, which is hot at its core and coo ...
... are in. If you leave the room, some small buoyancy-driven (or convective) motion of the air will continue because the walls can never be perfectly isothermal. Such processes go on in all plant and animal life and in the air around us. They occur throughout the earth, which is hot at its core and coo ...
Solutions to Exercises
... 6.31 a. The heat lost by the metal is equal to the heat gained by the water. Since q = s x m x t, the heat gained by the water is directly proportional to t. Since t is larger for metal A, it lost more heat. Now, each metal has the same mass and t, so the specific heat is directly proportional t ...
... 6.31 a. The heat lost by the metal is equal to the heat gained by the water. Since q = s x m x t, the heat gained by the water is directly proportional to t. Since t is larger for metal A, it lost more heat. Now, each metal has the same mass and t, so the specific heat is directly proportional t ...
Refrigeration and Air Conditioning Prof. M. Ramgopal Department of
... system undergoing an isentropic process one to two. So the work output is given by m into u one minus u two which can be written as m c v into T one minus T two where m is the mass of the system undergoing a the isentropic process. U one and u two are the specific internal energies at the beginning ...
... system undergoing an isentropic process one to two. So the work output is given by m into u one minus u two which can be written as m c v into T one minus T two where m is the mass of the system undergoing a the isentropic process. U one and u two are the specific internal energies at the beginning ...
Thermodynamics By S K Mondal
... 12. Two blocks which are at different states are brought into contact with each other and allowed to reach a final state of thermal equilibrium. The final temperature attained is specified by the (a) Zeroth law of thermodynamics (b) First law of thermodynamics [IES-1998] (c) Second law of thermodyn ...
... 12. Two blocks which are at different states are brought into contact with each other and allowed to reach a final state of thermal equilibrium. The final temperature attained is specified by the (a) Zeroth law of thermodynamics (b) First law of thermodynamics [IES-1998] (c) Second law of thermodyn ...
Chapter 4 - Aerostudents
... educators for course preparation. If you are a student using this Manual, you are using it without permission. ...
... educators for course preparation. If you are a student using this Manual, you are using it without permission. ...
Heat and Mass Transfer
... In this equation, a is the thermal diffusivity and q̇G is the internal heat generation per unit volume. Some problems, typically steady-state, one-dimensional formulations where only the heat flux is desired, can be solved simply from Equation (4.1.1). Most conduction analyses are performed with Equ ...
... In this equation, a is the thermal diffusivity and q̇G is the internal heat generation per unit volume. Some problems, typically steady-state, one-dimensional formulations where only the heat flux is desired, can be solved simply from Equation (4.1.1). Most conduction analyses are performed with Equ ...
Energy Generation and Conservation
... arrangement and motion of the molecules. It usually represented by U. E (energy) = PE + KE + U Law of conservation of energy: It states, “The energy can neither be created nor destroyed, through it can be transformed from one form to any other form, in which the energy can exist”. Heat: The heat is ...
... arrangement and motion of the molecules. It usually represented by U. E (energy) = PE + KE + U Law of conservation of energy: It states, “The energy can neither be created nor destroyed, through it can be transformed from one form to any other form, in which the energy can exist”. Heat: The heat is ...
Chapter 8: Exergy: A Measure of Work Potential
... increase. The exergy change of a system can be positive or negative during a process, but exergy destroyed cannot be negative. The decrease of exergy principle can be summarized as follows: ...
... increase. The exergy change of a system can be positive or negative during a process, but exergy destroyed cannot be negative. The decrease of exergy principle can be summarized as follows: ...
Knowledge Check (Answer Key)
... large amounts of energy transferred during a phase change from liquid to vapor or from vapor to liquid. This section will introduce thermodynamic terms that will be used to describe the various states and processes that water goes through when making a phase change. Classification of Properties Prop ...
... large amounts of energy transferred during a phase change from liquid to vapor or from vapor to liquid. This section will introduce thermodynamic terms that will be used to describe the various states and processes that water goes through when making a phase change. Classification of Properties Prop ...
Thermodynamics
... An important concept in thermodynamics is the thermodynamic system, a precisely defined region of the universe under study. Everything in the universe except the system is known as the surroundings. A system is separated from the remainder of the universe by a boundary which may be notional or not, b ...
... An important concept in thermodynamics is the thermodynamic system, a precisely defined region of the universe under study. Everything in the universe except the system is known as the surroundings. A system is separated from the remainder of the universe by a boundary which may be notional or not, b ...
Heat transfer

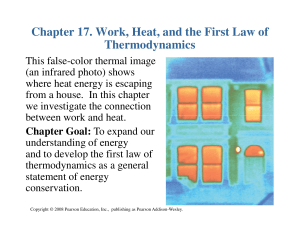
Heat transfer is the exchange of thermal energy between physical systems, depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation.Heat transfer always occurs from a region of high temperature to another region of lower temperature. Heat transfer changes the internal energy of both systems involved according to the First Law of Thermodynamics. The Second Law of Thermodynamics defines the concept of thermodynamic entropy, by measurable heat transfer.Thermal equilibrium is reached when all involved bodies and the surroundings reach the same temperature. Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.