Second Law of Thermodynamics
... 4.3 A generalized statement of the second law (review) Calculation of entropy requires an equivalent reversible process. [But all natural processes are irreversible since they move a system from a nonequilibrium state toward a condition of equilibrium.] The second law can be stated more generally in ...
... 4.3 A generalized statement of the second law (review) Calculation of entropy requires an equivalent reversible process. [But all natural processes are irreversible since they move a system from a nonequilibrium state toward a condition of equilibrium.] The second law can be stated more generally in ...
Why do molecules form the way they do?
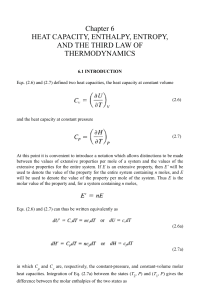
... - Exothermic reactions have negative DHrxn values -Typically (but not always) spontaneous reactions have negative values of DHrxn (the heat term is added to the products side) - We express enthalpy for a chemical reaction (DHrxn) as a stoichiometric component in a thermochemical equation 2H2O(l) 2 ...
... - Exothermic reactions have negative DHrxn values -Typically (but not always) spontaneous reactions have negative values of DHrxn (the heat term is added to the products side) - We express enthalpy for a chemical reaction (DHrxn) as a stoichiometric component in a thermochemical equation 2H2O(l) 2 ...
Midterm 2: Tue Nov 15 (Chs 11, 13, 14, 15, 19, 20)
... When a bimetallic bar made of copper and iron strips is heated, the bar bends toward the iron strip. The reason for this is A) iron expands more than copper. B) copper expands more than iron. C) copper gets hotter before iron. D) iron gets hotter before copper. E) none of these ...
... When a bimetallic bar made of copper and iron strips is heated, the bar bends toward the iron strip. The reason for this is A) iron expands more than copper. B) copper expands more than iron. C) copper gets hotter before iron. D) iron gets hotter before copper. E) none of these ...
Max Planck: the reluctant revolutionary Helge Kaigh, Physics World
... process known as "quantization". In his seminal paper published in late 1900 and presented to the German Physical Society on 14 December - 100 years ago this month - Planck regarded the energy "as made up of a completely determinate number of …nite equal parts, and for this purpose I use the constan ...
... process known as "quantization". In his seminal paper published in late 1900 and presented to the German Physical Society on 14 December - 100 years ago this month - Planck regarded the energy "as made up of a completely determinate number of …nite equal parts, and for this purpose I use the constan ...
Full text in PDF form
... similar to the states of stress in an elastically distended body. For at that time this was the only way one could conceive of only states that were apparently continuously distributed in space. The peculiar type of mechanical interpretation of these fields remained in the background--a sort of plac ...
... similar to the states of stress in an elastically distended body. For at that time this was the only way one could conceive of only states that were apparently continuously distributed in space. The peculiar type of mechanical interpretation of these fields remained in the background--a sort of plac ...
Statistical Physics Problem Sets 3–4: Kinetic Theory xford hysics
... b) Show that the average value of cos θ for these molecules is 23 . c) Using the results of Q3.3, show that for a gas obeying the Maxwellian distribution, the average energy of all the molecules is (3/2)kB T , but the average energy of those hitting the surface is 2kB T . 3.6 a) A Maxwellian gas eff ...
... b) Show that the average value of cos θ for these molecules is 23 . c) Using the results of Q3.3, show that for a gas obeying the Maxwellian distribution, the average energy of all the molecules is (3/2)kB T , but the average energy of those hitting the surface is 2kB T . 3.6 a) A Maxwellian gas eff ...
PH 2503 - Loyola College
... 5. What do you mean by equation of continuity? 6. State Graham’s Law of diffusion of gases. 7. What are generalized coordinates? What is the advantage of using them? 8. State the principle of virtual work. 9. Define Gravitation constant and mention its unit. ...
... 5. What do you mean by equation of continuity? 6. State Graham’s Law of diffusion of gases. 7. What are generalized coordinates? What is the advantage of using them? 8. State the principle of virtual work. 9. Define Gravitation constant and mention its unit. ...
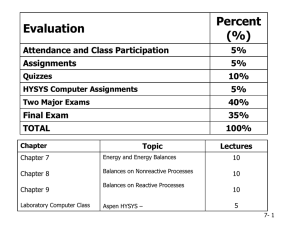
chapter20 - HCC Learning Web
... A connection was found between the transfer of energy by heat in thermal processes and the transfer of energy by work in mechanical processes. The concept of energy was generalized to include internal energy. The principle of conservation of energy emerged as a universal law of nature. ...
... A connection was found between the transfer of energy by heat in thermal processes and the transfer of energy by work in mechanical processes. The concept of energy was generalized to include internal energy. The principle of conservation of energy emerged as a universal law of nature. ...
Chemistry Entropy Notes 1. What is entropy? How many ways can
... system maintains the same outward appearance? This number of arrangements is called the entropy of the system. Actually, because the number of arrangements of atoms/molecules/ions is huge, entropy (S) is defined as the natural logarithm of the number of arrangements (W) multiplied by a constant (k): ...
... system maintains the same outward appearance? This number of arrangements is called the entropy of the system. Actually, because the number of arrangements of atoms/molecules/ions is huge, entropy (S) is defined as the natural logarithm of the number of arrangements (W) multiplied by a constant (k): ...
Grado - Universidad Pablo de Olavide, de Sevilla
... 3.To master the concepts of reaction rate and rate constant, as well as to know how to identify the factors that influence these magnitudes. To be able to describe proton-transfer and electrontransfer chemical reactions, applying thermodynamic concepts. 4.To know the basic principles of Surface Chem ...
... 3.To master the concepts of reaction rate and rate constant, as well as to know how to identify the factors that influence these magnitudes. To be able to describe proton-transfer and electrontransfer chemical reactions, applying thermodynamic concepts. 4.To know the basic principles of Surface Chem ...
Physical Science
... number of trials are constructed and interpreted; h) data tables for descriptive statistics showing specific measures of central tendency, the range of the data set, and the number of repeated trials are constructed and interpreted; i) frequency distributions, scatterplots, line plots, and histogram ...
... number of trials are constructed and interpreted; h) data tables for descriptive statistics showing specific measures of central tendency, the range of the data set, and the number of repeated trials are constructed and interpreted; i) frequency distributions, scatterplots, line plots, and histogram ...