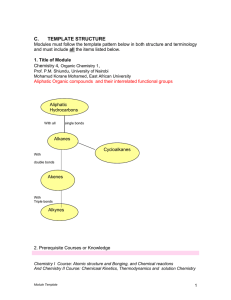
Reduction and Emergence in Chemistry
... the classical theory could not, namely the power to predict how two elements might react together. Or is McLaughlin suggesting that using quantum mechanics we can predict the properties of an element from a knowledge of the number of fundamental particles that its atoms possess? Unfortunately, as an ...
... the classical theory could not, namely the power to predict how two elements might react together. Or is McLaughlin suggesting that using quantum mechanics we can predict the properties of an element from a knowledge of the number of fundamental particles that its atoms possess? Unfortunately, as an ...
AP Chemistry Review Preparing for the AP
... Focus on your weakest areas; it is doubtful you can do/know everything. The AP Chemistry Exam is designed so that it is impossible to know absolutely everything on it (in case you haven’t noticed). I might as well place the biggest two at the start – You need to review your incorrect MC from the Pra ...
... Focus on your weakest areas; it is doubtful you can do/know everything. The AP Chemistry Exam is designed so that it is impossible to know absolutely everything on it (in case you haven’t noticed). I might as well place the biggest two at the start – You need to review your incorrect MC from the Pra ...
Class XI Physical Chemistry Short note
... Until 1920 an atom was supposed to consist of only 2 fundamental particles i.e. electrons and protons. Since electrons have negligible mass, the entire mass of the atom was regarded as the mass of the proton only. Each proton has a mass of 1.67x 10-24 g which is taken as 1 unit mass. In 1920, Ruther ...
... Until 1920 an atom was supposed to consist of only 2 fundamental particles i.e. electrons and protons. Since electrons have negligible mass, the entire mass of the atom was regarded as the mass of the proton only. Each proton has a mass of 1.67x 10-24 g which is taken as 1 unit mass. In 1920, Ruther ...
Head-Gordon`s
... techniques in a robust and reliable fashion to solve chemical problems? To the extent you can, then what are the unsolved issues in the field, or should it be considered a mature area where few fundamental challenges remain? To the extent you cannot, then what of significance has been accomplished o ...
... techniques in a robust and reliable fashion to solve chemical problems? To the extent you can, then what are the unsolved issues in the field, or should it be considered a mature area where few fundamental challenges remain? To the extent you cannot, then what of significance has been accomplished o ...
AP Chemistry: Bonding Multiple Choice
... (B) temperature at which the vapor pressure of the liquid is equal to 760 mm Hg. (C) temperature at which the solid, liquid, and vapor phases are all in equilibrium. (D) Temperature at which liquid and vapor phases are in equilibrium at I atmosphere. (E) lowest temperature above which a substance c ...
... (B) temperature at which the vapor pressure of the liquid is equal to 760 mm Hg. (C) temperature at which the solid, liquid, and vapor phases are all in equilibrium. (D) Temperature at which liquid and vapor phases are in equilibrium at I atmosphere. (E) lowest temperature above which a substance c ...
practice test 4 CHM 112
... 20. In the coordination compound [Cr(NH3)2(en)Cl2]Br2, the coordination number (C.N.) and oxidation number (O.N.) of the metal atom, respectively are A. C.N. = 6; O.N. = +4. B. C.N. = 6; O.N. = +3. C. C.N. = 5; O.N. = +2. D. C.N. = 5; O.N. = +4. E. C.N. = 4; O.N. = +3. ...
... 20. In the coordination compound [Cr(NH3)2(en)Cl2]Br2, the coordination number (C.N.) and oxidation number (O.N.) of the metal atom, respectively are A. C.N. = 6; O.N. = +4. B. C.N. = 6; O.N. = +3. C. C.N. = 5; O.N. = +2. D. C.N. = 5; O.N. = +4. E. C.N. = 4; O.N. = +3. ...
Section 5
... will depend on the polarity of the H-X bond (in most Brønsted acids, X = N, O, or a halogen) Electron-withdrawing groups attached to X will increase the quantity of partial positive charge on the H-atom, making it more susceptible to nucleophilic attack by a solvent (inductive effect) ...
... will depend on the polarity of the H-X bond (in most Brønsted acids, X = N, O, or a halogen) Electron-withdrawing groups attached to X will increase the quantity of partial positive charge on the H-atom, making it more susceptible to nucleophilic attack by a solvent (inductive effect) ...
The d-Block Elements
... The electronegativities of the first-row transition metals increase smoothly from Sc (χ = 1.4) to Cu (χ = 1.9). Thus Sc is a rather active metal, whereas Cu is much less reactive. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E° for the reactio ...
... The electronegativities of the first-row transition metals increase smoothly from Sc (χ = 1.4) to Cu (χ = 1.9). Thus Sc is a rather active metal, whereas Cu is much less reactive. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E° for the reactio ...
ATOMIC THEORY
... masses. Atoms of an element that are chemically alike but differ in mass are called ______________________ of the element. Because of the discovery of isotopes, scientists hypothesized that atoms contained still a third type of particle that explained these differences in mass. Calculations showed t ...
... masses. Atoms of an element that are chemically alike but differ in mass are called ______________________ of the element. Because of the discovery of isotopes, scientists hypothesized that atoms contained still a third type of particle that explained these differences in mass. Calculations showed t ...
Textbook Answer Keys - Mr. Massey`s Chemistry Pages
... shorter (smaller) the wavelength and the higher the frequency; ultraviolet light is high energy/ short wavelength when compared to visible light; infrared light is lower energy/low frequency compare to visible light; 7. B; the series of lines found in the visible region of the spectrum is called the ...
... shorter (smaller) the wavelength and the higher the frequency; ultraviolet light is high energy/ short wavelength when compared to visible light; infrared light is lower energy/low frequency compare to visible light; 7. B; the series of lines found in the visible region of the spectrum is called the ...
“No Score” from Exam 1??
... Multiple Bond and Bond Angles ! greater electron density on one side of the central atom ! Therefore, bond angles involving multiple bond are _____________, while angles on other side of ...
... Multiple Bond and Bond Angles ! greater electron density on one side of the central atom ! Therefore, bond angles involving multiple bond are _____________, while angles on other side of ...
Hands-On Chemistry Unit
... with one another, or how they combine or break apart, the total mass of the system remains the same. Understand that the atomic theory explains the conservation of matter: if the number of atoms stays the same no matter how they are rearranged, then their total mass stays the same. Explain that ener ...
... with one another, or how they combine or break apart, the total mass of the system remains the same. Understand that the atomic theory explains the conservation of matter: if the number of atoms stays the same no matter how they are rearranged, then their total mass stays the same. Explain that ener ...
2 - mrstorie
... a) *ionic bond – 3 bonding / 0 lone = trigonal planar b) 4 bonding/ 0 lone = tetrahedral c) 3 bonding/ 1 lone = trigonal pyramidal d) 6 bonding/ 0 lone = octahedral (*eO) e) 2 bonding / 2 lone = bent ...
... a) *ionic bond – 3 bonding / 0 lone = trigonal planar b) 4 bonding/ 0 lone = tetrahedral c) 3 bonding/ 1 lone = trigonal pyramidal d) 6 bonding/ 0 lone = octahedral (*eO) e) 2 bonding / 2 lone = bent ...
modeling secondary electron trajectories in scanning electron
... The efficiency of secondary electron collection by a scanning electron microscope detector is not generally known, particularly as the electric field on the detector is varied. It is often assumed that the detector collects almost all of the secondary electrons emitted from the sample. This works se ...
... The efficiency of secondary electron collection by a scanning electron microscope detector is not generally known, particularly as the electric field on the detector is varied. It is often assumed that the detector collects almost all of the secondary electrons emitted from the sample. This works se ...
Chemistry - Ysgol Bro Pedr
... possible to explain a fairly complex reaction using symbols and formulae, whilst telling us the ratio in which the atoms or molecules react. For example, sodium (a solid), reacts with water (a liquid with no solid dissolved in it) to form sodium hydroxide solution (solid sodium hydroxide dissolved i ...
... possible to explain a fairly complex reaction using symbols and formulae, whilst telling us the ratio in which the atoms or molecules react. For example, sodium (a solid), reacts with water (a liquid with no solid dissolved in it) to form sodium hydroxide solution (solid sodium hydroxide dissolved i ...
Scandium and Yttrium - Mercyhurst University
... reaction.4 At room temperature, scandium metal turns a light yellow or pink color in air.1 It is not obvious what is causing the color as Sc3+ would be expected to be colorless (white) due to the lack of d-electrons. Purification and Properties The most interesting thing about these two elements is ...
... reaction.4 At room temperature, scandium metal turns a light yellow or pink color in air.1 It is not obvious what is causing the color as Sc3+ would be expected to be colorless (white) due to the lack of d-electrons. Purification and Properties The most interesting thing about these two elements is ...
1 - Cathedral High School
... 7.2.4 Explain that reactions can occur by more than one step and that one step can determine the rate of reaction. Few reactions involve just one step although one step in the reaction, the rate determining step, determines the reaction rate. Orders of reactions and rate ...
... 7.2.4 Explain that reactions can occur by more than one step and that one step can determine the rate of reaction. Few reactions involve just one step although one step in the reaction, the rate determining step, determines the reaction rate. Orders of reactions and rate ...
Chemistry 2008 Multiple Choice
... in a 1 to 1 ratio, thus three moles of HCl would require 3 moles of O2, which is what is available. c. (1) K2O + H2O 2 K+ + 2OH- ...
... in a 1 to 1 ratio, thus three moles of HCl would require 3 moles of O2, which is what is available. c. (1) K2O + H2O 2 K+ + 2OH- ...
SQA CfE Higher Chemistry Unit 1: Chemical Changes and Structure
... All substances are made up of particles called atoms, ions or molecules, and these particles are constantly moving. The degree of movement depends upon the state of the substance. This is known as the "kinetic model" of matter. In any sample of solution, liquid or gas there is a range of kinetic ene ...
... All substances are made up of particles called atoms, ions or molecules, and these particles are constantly moving. The degree of movement depends upon the state of the substance. This is known as the "kinetic model" of matter. In any sample of solution, liquid or gas there is a range of kinetic ene ...
chemistry
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
2.6 M - Thierry Karsenti
... 3. If another functional group is present which IUPAC rules prescribe must be named as a suffix, the aldehyde group is named with the prefix formyl-. This prefix is preferred to methanoyl-. Aldehydes are also commonly recognised as alkanals, for example, methanal and ethanal are the basic ...
... 3. If another functional group is present which IUPAC rules prescribe must be named as a suffix, the aldehyde group is named with the prefix formyl-. This prefix is preferred to methanoyl-. Aldehydes are also commonly recognised as alkanals, for example, methanal and ethanal are the basic ...
As a result of activities in grades 9
... water. Enthalpy of formation. "Special reactions" like combustion, electrolysis, photosynthesis, and respiration. Many other topics are covered in less detail. For example, the graduate will gain an understanding of the fundamentals of pH but not to the depths required for calculating it (because th ...
... water. Enthalpy of formation. "Special reactions" like combustion, electrolysis, photosynthesis, and respiration. Many other topics are covered in less detail. For example, the graduate will gain an understanding of the fundamentals of pH but not to the depths required for calculating it (because th ...
TEKS Presentation Properties of Matter
... How the specific heat of water affects the Earth Oceans cover about 2/3 of Earth’s surface. Water’s characteristic of retaining heat is important to our climate. It means that our climate stays much more stable than it would if there were less water on Earth. TAKS Need to Know ...
... How the specific heat of water affects the Earth Oceans cover about 2/3 of Earth’s surface. Water’s characteristic of retaining heat is important to our climate. It means that our climate stays much more stable than it would if there were less water on Earth. TAKS Need to Know ...
Minerals - UNLV Geoscience
... Structure of minerals Polymorphs • Two or more minerals with the same chemical composition but different crystalline structures • Diamond and graphite are good examples of polymorphs – The transformation of one polymorph to another is called a phase change ...
... Structure of minerals Polymorphs • Two or more minerals with the same chemical composition but different crystalline structures • Diamond and graphite are good examples of polymorphs – The transformation of one polymorph to another is called a phase change ...
Chapter 1: Matter and Measurement
... Read atomic masses. Read the ions formed by main group elements. Read the electron configuration. Learn trends in physical and chemical properties. ...
... Read atomic masses. Read the ions formed by main group elements. Read the electron configuration. Learn trends in physical and chemical properties. ...