9 SHS CH 9 LECTURE shs_ch_9_lecture
... not in mole units convert it to moles using the molecular mass as a conversion factor. Step 3. Now convert from the moles of starting substance to the moles of the desired substance by using a mole ratio from the chemical reaction. reaction. Step 4. If the problem is to find grams of the substance, ...
... not in mole units convert it to moles using the molecular mass as a conversion factor. Step 3. Now convert from the moles of starting substance to the moles of the desired substance by using a mole ratio from the chemical reaction. reaction. Step 4. If the problem is to find grams of the substance, ...
CHAPTER 4 REACTIONS IN AQUEOUS SOLUTIONS
... In order to work this problem, you need to assign the oxidation numbers to all the elements in the compounds. In each case oxygen has an oxidation number of −2 (rule 3). These oxidation numbers should then be compared to the range of possible oxidation numbers that each element can have. Molecular o ...
... In order to work this problem, you need to assign the oxidation numbers to all the elements in the compounds. In each case oxygen has an oxidation number of −2 (rule 3). These oxidation numbers should then be compared to the range of possible oxidation numbers that each element can have. Molecular o ...
Soot Formation Modeling during Hydrocarbon
... species with pre-existing soot surface can be an important factor for the particle mass growth. Following these conclusions, another detailed kinetic model was developed (Model-2), where PAH were considered as soot precursors, and aliphatic species take place only in reactions of surface growth. Bot ...
... species with pre-existing soot surface can be an important factor for the particle mass growth. Following these conclusions, another detailed kinetic model was developed (Model-2), where PAH were considered as soot precursors, and aliphatic species take place only in reactions of surface growth. Bot ...
chapter 20 - Chemistry
... The species that can oxidize water to molecular oxygen must have an Ered more positive than 1.23 V. From Table 18.1 of the text we see that only Cl2(g) and MnO4 (aq ) in acid solution can oxidize water to oxygen. ...
... The species that can oxidize water to molecular oxygen must have an Ered more positive than 1.23 V. From Table 18.1 of the text we see that only Cl2(g) and MnO4 (aq ) in acid solution can oxidize water to oxygen. ...
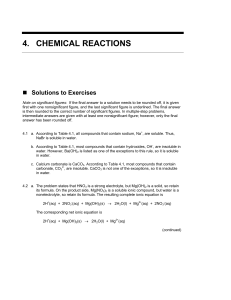
4. chemical reactions
... must equal the moles of acid in flask A. You take the number of moles of NaOH and divide it by the volume of NaOH added during the titration to determine the concentration of the NaOH solution. ...
... must equal the moles of acid in flask A. You take the number of moles of NaOH and divide it by the volume of NaOH added during the titration to determine the concentration of the NaOH solution. ...
Chapter 4 - Chemistry
... (a) is a strong electrolyte. The compound dissociates completely into ions in solution. (b) is a nonelectrolyte. The compound dissolves in water, but the molecules remain intact. (c) is a weak electrolyte. A small amount of the compound dissociates into ions in water. When NaCl dissolves in water it ...
... (a) is a strong electrolyte. The compound dissociates completely into ions in solution. (b) is a nonelectrolyte. The compound dissolves in water, but the molecules remain intact. (c) is a weak electrolyte. A small amount of the compound dissociates into ions in water. When NaCl dissolves in water it ...
Announcements - University of Illinois Urbana
... removal, so reactor heats up until a steady state is reached • R(T) > G(T) (R(T) line above G(T) on graph): rate of heat generation < heat removal, so reactor cools off until a steady state is reached Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urban ...
... removal, so reactor heats up until a steady state is reached • R(T) > G(T) (R(T) line above G(T) on graph): rate of heat generation < heat removal, so reactor cools off until a steady state is reached Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urban ...
Stoichiometric Calculations
... Stoichiometry is the quantitative determination of reactants or products using a balanced chemical equation. We can interpret a balanced equation in several ways. The most common way is to interpret it as indicating the number of moles of each substance. For instance, this equation, 3 H2 + N2 ® 2 NH ...
... Stoichiometry is the quantitative determination of reactants or products using a balanced chemical equation. We can interpret a balanced equation in several ways. The most common way is to interpret it as indicating the number of moles of each substance. For instance, this equation, 3 H2 + N2 ® 2 NH ...
chapter 2 - chemical equations and reaction yields
... The least product comes from the supply of tomato slices. That will make 250 cheeseburgers. For these, 500 hamburger patties are required, so 100 hamburger patties will be left over. ...
... The least product comes from the supply of tomato slices. That will make 250 cheeseburgers. For these, 500 hamburger patties are required, so 100 hamburger patties will be left over. ...
Chapter 1: Matter and Measurements
... Chapter 1: Matter and Measurements 22. Refer to Section 1.2. (a) 4020.6 mL = 4.0206 x 103 mL (b) 1.006 g (This is already in proper scientific notation.) (c) 100.1°C = 1.001 x 102°C 32. Refer to Section 1.2 and Table 1.2. Convert one of the numbers to the units of the other. Once the numbers are exp ...
... Chapter 1: Matter and Measurements 22. Refer to Section 1.2. (a) 4020.6 mL = 4.0206 x 103 mL (b) 1.006 g (This is already in proper scientific notation.) (c) 100.1°C = 1.001 x 102°C 32. Refer to Section 1.2 and Table 1.2. Convert one of the numbers to the units of the other. Once the numbers are exp ...
Chapter 4 - UCF Chemistry
... Nickel forms a compound with CO, Ni(CO)x. To determine its formula, you carefully heat a 0.0973-g sample in air to convert the Ni in 0.0426 g NiO and the CO in 0.100 g of CO2. What is the empirical formula of Ni(CO)x? From moles of NiO and CO2 we can calculate moles of Ni and CO: molar mass of NiO = ...
... Nickel forms a compound with CO, Ni(CO)x. To determine its formula, you carefully heat a 0.0973-g sample in air to convert the Ni in 0.0426 g NiO and the CO in 0.100 g of CO2. What is the empirical formula of Ni(CO)x? From moles of NiO and CO2 we can calculate moles of Ni and CO: molar mass of NiO = ...
2 - Chemistry
... Nickel forms a compound with CO, Ni(CO)x. To determine its formula, you carefully heat a 0.0973-g sample in air to convert the Ni in 0.0426 g NiO and the CO in 0.100 g of CO2. What is the empirical formula of Ni(CO)x? From moles of NiO and CO2 we can calculate moles of Ni and CO: molar mass of NiO = ...
... Nickel forms a compound with CO, Ni(CO)x. To determine its formula, you carefully heat a 0.0973-g sample in air to convert the Ni in 0.0426 g NiO and the CO in 0.100 g of CO2. What is the empirical formula of Ni(CO)x? From moles of NiO and CO2 we can calculate moles of Ni and CO: molar mass of NiO = ...
Question Bank (Class XI - Chemistry)
... Q3- What is a chemical equation? What are its essential features? (L-2) Ans. the qualitative and quantitative representation of a chemical reaction in short form in terms of symbols and formulae is called chemical equation. For example, on heating calcium carbonate, it gives Caco3 →Ca0 + CO2 Essenti ...
... Q3- What is a chemical equation? What are its essential features? (L-2) Ans. the qualitative and quantitative representation of a chemical reaction in short form in terms of symbols and formulae is called chemical equation. For example, on heating calcium carbonate, it gives Caco3 →Ca0 + CO2 Essenti ...
Atmospheric evolution in the Precambrian: Constraints from water
... The effects of Po2 on mineral dissolution have been studied for Fe(II)-bearing silicate minerals (Murakami et al., 2004; Sugimori et al., 2009, 2012). Although the observation of redox-insensitive elements (e.g., Mg and Si) released during dissolution of the minerals has revealed that dissolution ra ...
... The effects of Po2 on mineral dissolution have been studied for Fe(II)-bearing silicate minerals (Murakami et al., 2004; Sugimori et al., 2009, 2012). Although the observation of redox-insensitive elements (e.g., Mg and Si) released during dissolution of the minerals has revealed that dissolution ra ...
HW 19
... Strategy: The standard emf (E°) can be calculated using the standard reduction potentials in Table 19.1 of the text. Because the reactions are not run under standard-state conditions (concentrations are not 1 M), we need Nernst's equation [Equation (19.8) of the text] to calculate the emf (E) of a h ...
... Strategy: The standard emf (E°) can be calculated using the standard reduction potentials in Table 19.1 of the text. Because the reactions are not run under standard-state conditions (concentrations are not 1 M), we need Nernst's equation [Equation (19.8) of the text] to calculate the emf (E) of a h ...
Problem Set 7
... this previous statement true if CO2 has a different size than radon, but both are gases? The conditions at STP are 0oC and 1 atm of pressure. CO2 and Rn are certainly different sized particles, but because gases have no intermolecular attraction and very high kinetic energy, the volume they occupy a ...
... this previous statement true if CO2 has a different size than radon, but both are gases? The conditions at STP are 0oC and 1 atm of pressure. CO2 and Rn are certainly different sized particles, but because gases have no intermolecular attraction and very high kinetic energy, the volume they occupy a ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.