Lesson 1 Reversible reactions and equilibrium
... 4. Grow a quick crop of legumes – good But depends on timings of crops, etc. 5. Use fertiliser with larger particle size – good Using the same amount of fertiliser with larger particles is likely to lead to a slower dissolving into the ground, giving plants more time to absorb it. ...
... 4. Grow a quick crop of legumes – good But depends on timings of crops, etc. 5. Use fertiliser with larger particle size – good Using the same amount of fertiliser with larger particles is likely to lead to a slower dissolving into the ground, giving plants more time to absorb it. ...
Reaction Rate Graphs C12-3
... Ineffective collisions involve particles that rebound essentially unchanged. The rate of reaction depends upon the frequency of collisions and the fractions of those collisions that are effective. Reaction Rate is measured as a decrease in the concentration of reactants per unit time or an incre ...
... Ineffective collisions involve particles that rebound essentially unchanged. The rate of reaction depends upon the frequency of collisions and the fractions of those collisions that are effective. Reaction Rate is measured as a decrease in the concentration of reactants per unit time or an incre ...
Page|1 - askIITians
... system. For example, hot coffee in an open flask because it can gain or loose matter and energy with the surroundings. Q9. Pressure on the system remains constant in adiabatic process isobaric process isochroic process reversible process ...
... system. For example, hot coffee in an open flask because it can gain or loose matter and energy with the surroundings. Q9. Pressure on the system remains constant in adiabatic process isobaric process isochroic process reversible process ...
Fundamental Knowledge for Analysis of Chemical Reactor
... How long a reaction time will be? • Example: Decomposition of di-tert-butyl peroxide (CH3)3COOC(CH3)2C2H6 + 2CH3COCH3 • What is the reaction rate: r = -dN/dt • How long a time for a given conversion: t = N0N(-dN/r) • Obviously, r = r(N) ...
... How long a reaction time will be? • Example: Decomposition of di-tert-butyl peroxide (CH3)3COOC(CH3)2C2H6 + 2CH3COCH3 • What is the reaction rate: r = -dN/dt • How long a time for a given conversion: t = N0N(-dN/r) • Obviously, r = r(N) ...
Chemical Reactions (L1)
... form (Fe), and substance “B” is an element in molecular form (O2). The result is a direct chemical combination of the two elements (FeO, iron oxide, which is “rust”). ...
... form (Fe), and substance “B” is an element in molecular form (O2). The result is a direct chemical combination of the two elements (FeO, iron oxide, which is “rust”). ...
Chapters 12 – 20 Practice Problems
... 17. For the reaction N2O4(g) ↔ 2 NO2(g) the value of Kc = 1.07 x 10-5. If the initial concentrations of N2O4 is 0.0125 M, what will be the equilibrium concentration of [NO2]? A) 3.66 x 10-4 M ...
... 17. For the reaction N2O4(g) ↔ 2 NO2(g) the value of Kc = 1.07 x 10-5. If the initial concentrations of N2O4 is 0.0125 M, what will be the equilibrium concentration of [NO2]? A) 3.66 x 10-4 M ...
Slide 1
... Ideal Redox Sequence There is an ideal sequence of redox reactions driven by e- rich organic matter that is based on the energy available for the microbes that mediate the reactions. ...
... Ideal Redox Sequence There is an ideal sequence of redox reactions driven by e- rich organic matter that is based on the energy available for the microbes that mediate the reactions. ...
C6_rev - boswellsrcd
... Use simple collision theory to explain how rates of reaction depend on the concentration of solutions of soluble chemicals. • Reactions in solution involve dissolved particles that must collide before reaction is possible. • The more crowded (concentrated) the solution, the faster the reaction beca ...
... Use simple collision theory to explain how rates of reaction depend on the concentration of solutions of soluble chemicals. • Reactions in solution involve dissolved particles that must collide before reaction is possible. • The more crowded (concentrated) the solution, the faster the reaction beca ...
Types of Chemical Reactions (rxns.)
... A metal can replace a metal (+) OR a nonmetal can replace a nonmetal (-). element + compoundà product + product A + BC à AC + B (if A is a metal) OR A + BC à BA + C (if A is a nonmetal) (remember the cation always goes first!) ...
... A metal can replace a metal (+) OR a nonmetal can replace a nonmetal (-). element + compoundà product + product A + BC à AC + B (if A is a metal) OR A + BC à BA + C (if A is a nonmetal) (remember the cation always goes first!) ...
Ionic Equations
... sodium hydroxide and calcium bromide to give calcium hydroxide and sodium bromide ...
... sodium hydroxide and calcium bromide to give calcium hydroxide and sodium bromide ...
Conservation of Mass Lab
... during a chemical reaction. This means that all chemical reactions must be balanced—the number of atoms, moles, and ultimately the total mass must be conserved during a chemical process. Here are the rules to follow when balancing equations: ...
... during a chemical reaction. This means that all chemical reactions must be balanced—the number of atoms, moles, and ultimately the total mass must be conserved during a chemical process. Here are the rules to follow when balancing equations: ...
Test review
... 11a. increase, shift left 11b. increase, shift left 11c no change 11d. increase, shift left 11e. decrease, shift right 12. 2 x 103 molecules/cm3 13. FeSCN2+ = 2.0 M, Fe3+ and SCN- = 0.043 M 14. 134 atm-1 15. 2.1 x 10-3 atm 16. PP4 = 0.73 atm, PP2 = 0.270 atm, 16% P4 is dissociated 17. PNO2 = .71 atm ...
... 11a. increase, shift left 11b. increase, shift left 11c no change 11d. increase, shift left 11e. decrease, shift right 12. 2 x 103 molecules/cm3 13. FeSCN2+ = 2.0 M, Fe3+ and SCN- = 0.043 M 14. 134 atm-1 15. 2.1 x 10-3 atm 16. PP4 = 0.73 atm, PP2 = 0.270 atm, 16% P4 is dissociated 17. PNO2 = .71 atm ...
Exam 3 Review Key
... b) Lead’s primary mode of toxicity is its interference with enzyme function – it mimics other essential metals that take part in enzymatic reactions and displaces them. Considering the fact that sulfhydryl (-SH) groups are found on many enzymes, how might EDTA and DMSA work to treat lead poisoning? ...
... b) Lead’s primary mode of toxicity is its interference with enzyme function – it mimics other essential metals that take part in enzymatic reactions and displaces them. Considering the fact that sulfhydryl (-SH) groups are found on many enzymes, how might EDTA and DMSA work to treat lead poisoning? ...
Synthesis Reaction
... I can describe evidence of a chemical reaction from experimental observations. I can balance chemical equations to fulfill the Law of Conservation of Mass I can interpret changes in matter and energy from complete chemical equations I can write chemical reactions by interpreting word equations I can ...
... I can describe evidence of a chemical reaction from experimental observations. I can balance chemical equations to fulfill the Law of Conservation of Mass I can interpret changes in matter and energy from complete chemical equations I can write chemical reactions by interpreting word equations I can ...
Document
... I can describe evidence of a chemical reaction from experimental observations. I can balance chemical equations to fulfill the Law of Conservation of Mass I can interpret changes in matter and energy from complete chemical equations I can write chemical reactions by interpreting word equations I can ...
... I can describe evidence of a chemical reaction from experimental observations. I can balance chemical equations to fulfill the Law of Conservation of Mass I can interpret changes in matter and energy from complete chemical equations I can write chemical reactions by interpreting word equations I can ...
Reaction Analysis and PAT Tools
... useful and meaningful information about chemical reactions, in real time. The result of an extensive research project on how scientists analyze reactions, iC IR allows chemists and engineers to quickly gain an understanding of their chemistry. ReactIR collects data in the mid infrared spectral regio ...
... useful and meaningful information about chemical reactions, in real time. The result of an extensive research project on how scientists analyze reactions, iC IR allows chemists and engineers to quickly gain an understanding of their chemistry. ReactIR collects data in the mid infrared spectral regio ...
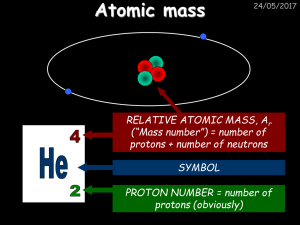
Atomic mass - drseemaljelani
... calculated amount of product. Because: • The reaction may not totally finish – it may be reversible • Some of the product may be lost when it is separated from the reaction mixture – filtered • Some of the reactants may react in different ways to the expected reaction ...
... calculated amount of product. Because: • The reaction may not totally finish – it may be reversible • Some of the product may be lost when it is separated from the reaction mixture – filtered • Some of the reactants may react in different ways to the expected reaction ...
Addition of ketene to ethylene oxide
... This Thesis - Open Access is brought to you for free and open access by Scholars' Mine. It has been accepted for inclusion in Masters Theses by an authorized administrator of Scholars' Mine. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution req ...
... This Thesis - Open Access is brought to you for free and open access by Scholars' Mine. It has been accepted for inclusion in Masters Theses by an authorized administrator of Scholars' Mine. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution req ...
Chemical Bonding (short)
... H = - 103 kJ and activation energy = 22 kJ 8. Sketch a generic PE diagram for an endothermic reaction. Label the reactants and products, the heat of reaction (∆H), and activation energy (Eact). 9. On your sketch above, draw a dotted line on your PE diagram and label it to show the effect of adding ...
... H = - 103 kJ and activation energy = 22 kJ 8. Sketch a generic PE diagram for an endothermic reaction. Label the reactants and products, the heat of reaction (∆H), and activation energy (Eact). 9. On your sketch above, draw a dotted line on your PE diagram and label it to show the effect of adding ...
Describing Chemical Reactions
... To describe a reaction accurately, a chemical equation must show the same number of each type of atom on both sides of the equation. An equation is balanced when it accurately represents conservation of mass. To balance a chemical equation, you may have to use coefficients. A coefficient is a number ...
... To describe a reaction accurately, a chemical equation must show the same number of each type of atom on both sides of the equation. An equation is balanced when it accurately represents conservation of mass. To balance a chemical equation, you may have to use coefficients. A coefficient is a number ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.