Preparation of Reducing Sugar Hydrolyzed from High
... This study aims to produce reducing sugar hydrolyzed from substrate, coconut coir dust pretreated by recycled ionic liquid and its combination with alkaline. The 1H NMR and FTIR were performed to verify the synthesized ionic liquid methylmethylimidazolium dimethyl phosphate ([mmim][dmp]). The struct ...
... This study aims to produce reducing sugar hydrolyzed from substrate, coconut coir dust pretreated by recycled ionic liquid and its combination with alkaline. The 1H NMR and FTIR were performed to verify the synthesized ionic liquid methylmethylimidazolium dimethyl phosphate ([mmim][dmp]). The struct ...
Stoichiometry and the Mole - 2012 Book Archive
... Although the number of things in a mole is known to eight decimal places, it is usually fine to use only two or three decimal places in calculations. The numerical value of things in a mole is often called Avogadro’s number (NA), which is also known as the Avogadro constant, after Amadeo Avogadro, a ...
... Although the number of things in a mole is known to eight decimal places, it is usually fine to use only two or three decimal places in calculations. The numerical value of things in a mole is often called Avogadro’s number (NA), which is also known as the Avogadro constant, after Amadeo Avogadro, a ...
5 How Chemists Measure Atoms and Molecules
... assign relative masses, called atomic weights, to atoms. They did this by comparing the masses of two elements that react with each other. Dalton chose hydrogen (assumed to have an atomic weight of 1) as the reference point for his scale of atomic weights. Scientists continue to use a scale of relat ...
... assign relative masses, called atomic weights, to atoms. They did this by comparing the masses of two elements that react with each other. Dalton chose hydrogen (assumed to have an atomic weight of 1) as the reference point for his scale of atomic weights. Scientists continue to use a scale of relat ...
5 How Chemists Measure Atoms and Molecules
... assign relative masses, called atomic weights, to atoms. They did this by comparing the masses of two elements that react with each other. Dalton chose hydrogen (assumed to have an atomic weight of 1) as the reference point for his scale of atomic weights. Scientists continue to use a scale of relat ...
... assign relative masses, called atomic weights, to atoms. They did this by comparing the masses of two elements that react with each other. Dalton chose hydrogen (assumed to have an atomic weight of 1) as the reference point for his scale of atomic weights. Scientists continue to use a scale of relat ...
5 How Chemists Measure Atoms and Molecules
... assign relative masses, called atomic weights, to atoms. They did this by comparing the masses of two elements that react with each other. Dalton chose hydrogen (assumed to have an atomic weight of 1) as the reference point for his scale of atomic weights. Scientists continue to use a scale of relat ...
... assign relative masses, called atomic weights, to atoms. They did this by comparing the masses of two elements that react with each other. Dalton chose hydrogen (assumed to have an atomic weight of 1) as the reference point for his scale of atomic weights. Scientists continue to use a scale of relat ...
Exam Review Packet Table of Contents
... -‐2,240 kilojoules per mole. Account for this difference. ...
... -‐2,240 kilojoules per mole. Account for this difference. ...
The roles of electronic exchange and correlation in charge
... over 2 eV. Even the use of a quantum mechanics/molecular mechanics 共QM/MM兲-like approach, where we calculated the electronic structure with our CISD method using solvent configurations generated from the one-electron simulations, still produced an absorption spectrum that was shifted ⬃1 eV to the bl ...
... over 2 eV. Even the use of a quantum mechanics/molecular mechanics 共QM/MM兲-like approach, where we calculated the electronic structure with our CISD method using solvent configurations generated from the one-electron simulations, still produced an absorption spectrum that was shifted ⬃1 eV to the bl ...
tailoringoxidepro
... Cu(111), which is used as a substrate to explore charge transport to nanostructures including Au adatoms24–26 . While the stoichiometry and atomic structure of ultra-thin MgO corresponds that of the bulk MgO, this is not always the case. The most prominent example of this is an ultra-thin alumina fi ...
... Cu(111), which is used as a substrate to explore charge transport to nanostructures including Au adatoms24–26 . While the stoichiometry and atomic structure of ultra-thin MgO corresponds that of the bulk MgO, this is not always the case. The most prominent example of this is an ultra-thin alumina fi ...
Chemistry HL Syllabus Details
... the carbon atom and the two oxygen atoms in the carboxyl group of a carboxylic acid. ...
... the carbon atom and the two oxygen atoms in the carboxyl group of a carboxylic acid. ...
Document
... carbon found on earth (natural carbon) is a mixture of the isotopes 12C, 13C, and 14C. – All three isotopes have six protons, but they have six, seven, and eight neutrons, respectively. Because natural carbon is a mixture of isotopes, the atomic mass we use for carbon is an average value based o ...
... carbon found on earth (natural carbon) is a mixture of the isotopes 12C, 13C, and 14C. – All three isotopes have six protons, but they have six, seven, and eight neutrons, respectively. Because natural carbon is a mixture of isotopes, the atomic mass we use for carbon is an average value based o ...
Synthesis and Structural Studies of Calcium and Magnesium
... The work presented herein describes synthetic methodologies leading to the design of a wide array of magnesium and calcium based phosphinate and phosphonates with possible applications as bone scaffolding materials or additives to bone cements. The challenge to the chemistry of the alkaline earth ph ...
... The work presented herein describes synthetic methodologies leading to the design of a wide array of magnesium and calcium based phosphinate and phosphonates with possible applications as bone scaffolding materials or additives to bone cements. The challenge to the chemistry of the alkaline earth ph ...
Stoichiometry and the Mole
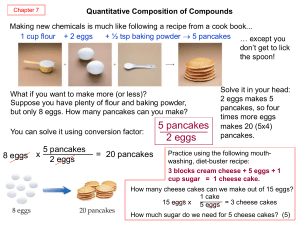
... Again, units cancel, and new units are introduced. A balanced chemical equation is nothing more than a recipe for a chemical reaction. The difference is that a balanced chemical equation is written in terms of atoms and molecules, not cups, pounds, and eggs. For example, consider the following chemic ...
... Again, units cancel, and new units are introduced. A balanced chemical equation is nothing more than a recipe for a chemical reaction. The difference is that a balanced chemical equation is written in terms of atoms and molecules, not cups, pounds, and eggs. For example, consider the following chemic ...
KCET – CHEMISTRY – 2016 - Medicine.careers360.com
... 12. Replacement of Cl of Chlorobenzene to give phenol requires drastic conditions, but Cl of 2, 4dinitro chlorobenene is readily replaced. This is because 1) –NO2 group makes the ring electron rich at ortho and para positions 2) –NO2 group withdraws electrons from meta position 3) –NO2donate electro ...
... 12. Replacement of Cl of Chlorobenzene to give phenol requires drastic conditions, but Cl of 2, 4dinitro chlorobenene is readily replaced. This is because 1) –NO2 group makes the ring electron rich at ortho and para positions 2) –NO2 group withdraws electrons from meta position 3) –NO2donate electro ...
Predictive thermodynamics for ionic solids and
... does not preclude formation of useful proportions of product which can be extracted from the reaction system).20 Furthermore, experimentally-derived thermodynamic values themselves can have considerable uncertainties.21–23 The actual mathematics required is minimal yet quantitative interpretation re ...
... does not preclude formation of useful proportions of product which can be extracted from the reaction system).20 Furthermore, experimentally-derived thermodynamic values themselves can have considerable uncertainties.21–23 The actual mathematics required is minimal yet quantitative interpretation re ...
Radical Cations in Mixtures of ClsP and Me#. A Combined ESR and
... and Me2S in frozen halocarbon solutions. The choice for this combination was initially based on the increased AIP (IP(C1,P) = 9.81 eV, Alp = 1.13 eV) compared to the Me3P and Me2S combination. Our interest in the system developed during the course of this study when a variety of radical cation produ ...
... and Me2S in frozen halocarbon solutions. The choice for this combination was initially based on the increased AIP (IP(C1,P) = 9.81 eV, Alp = 1.13 eV) compared to the Me3P and Me2S combination. Our interest in the system developed during the course of this study when a variety of radical cation produ ...
Few-body interactions in an ultracold gas of Cesium atoms
... research on and with Feshbach molecules is given in order to set this work in relation to ongoing research efforts. The third chapter gives a short outline of the binary interactions acting between Cs atoms and the resulting molecular energy structure just below threshold. Emphasis is given to the i ...
... research on and with Feshbach molecules is given in order to set this work in relation to ongoing research efforts. The third chapter gives a short outline of the binary interactions acting between Cs atoms and the resulting molecular energy structure just below threshold. Emphasis is given to the i ...
"Fundamentals of Electronic Spectroscopy" in
... an electronic state that can be described as a valence state at short internuclear distances, may evolve into a Rydberg state or an ion-pair state at larger distances, or even display shallow van der Waals potential wells. The complexity of the electronic structure of even the simplest molecular sys ...
... an electronic state that can be described as a valence state at short internuclear distances, may evolve into a Rydberg state or an ion-pair state at larger distances, or even display shallow van der Waals potential wells. The complexity of the electronic structure of even the simplest molecular sys ...
Document
... 1. Write and balance equation. 2. Calculate the number of moles of product for each reactant; 3. The reactant that gives the least moles of (the same!) product is the limiting reactant. 4. Find the amount of reactant in excess needed to react with the limiting reactant. Subtract this amount from the ...
... 1. Write and balance equation. 2. Calculate the number of moles of product for each reactant; 3. The reactant that gives the least moles of (the same!) product is the limiting reactant. 4. Find the amount of reactant in excess needed to react with the limiting reactant. Subtract this amount from the ...
c00kieee - Ritter Illustration
... It should be noted that at the creation of the universe some amount of 244 Pu could have been formed; however, with an 80 million year half-life, it would have fully decayed during the past 10 billion years. Uranium was the first actinide element to be identified. In 1789, M. H. Klaproth discovered ...
... It should be noted that at the creation of the universe some amount of 244 Pu could have been formed; however, with an 80 million year half-life, it would have fully decayed during the past 10 billion years. Uranium was the first actinide element to be identified. In 1789, M. H. Klaproth discovered ...
Chemistry In action
... This book was typeset in 10/12 Times New Roman at cMPreparé and printed and bound by R. R. Donnelley/Jefferson City. The cover was printed by R. R. Donnelley/Jefferson City. The paper in this book was manufactured by a mill whose forest management programs include sustained yield—harvesting of its t ...
... This book was typeset in 10/12 Times New Roman at cMPreparé and printed and bound by R. R. Donnelley/Jefferson City. The cover was printed by R. R. Donnelley/Jefferson City. The paper in this book was manufactured by a mill whose forest management programs include sustained yield—harvesting of its t ...
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic force of attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction. The strength of chemical bonds varies considerably; there are ""strong bonds"" such as covalent or ionic bonds and ""weak bonds"" such as Dipole-dipole interaction, the London dispersion force and hydrogen bonding.Since opposite charges attract via a simple electromagnetic force, the negatively charged electrons that are orbiting the nucleus and the positively charged protons in the nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitutes the chemical bond. Due to the matter wave nature of electrons and their smaller mass, they must occupy a much larger amount of volume compared with the nuclei, and this volume occupied by the electrons keeps the atomic nuclei relatively far apart, as compared with the size of the nuclei themselves. This phenomenon limits the distance between nuclei and atoms in a bond.In general, strong chemical bonding is associated with the sharing or transfer of electrons between the participating atoms. The atoms in molecules, crystals, metals and diatomic gases—indeed most of the physical environment around us—are held together by chemical bonds, which dictate the structure and the bulk properties of matter.All bonds can be explained by quantum theory, but, in practice, simplification rules allow chemists to predict the strength, directionality, and polarity of bonds. The octet rule and VSEPR theory are two examples. More sophisticated theories are valence bond theory which includes orbital hybridization and resonance, and the linear combination of atomic orbitals molecular orbital method which includes ligand field theory. Electrostatics are used to describe bond polarities and the effects they have on chemical substances.