experiment 18: flame tests for metals
... directly to fundamental concepts taught in basic chemistry classes. The media resources featured in this lesson provide a visually rich way to tie together spectral chemistry, combustion, and the nature of fire. Students watch a video segment and read text about the color of fireworks (particularly ...
... directly to fundamental concepts taught in basic chemistry classes. The media resources featured in this lesson provide a visually rich way to tie together spectral chemistry, combustion, and the nature of fire. Students watch a video segment and read text about the color of fireworks (particularly ...
Exam 2
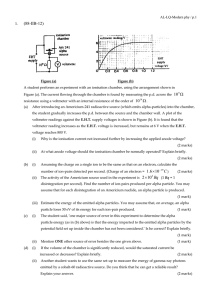
... unsimpliÞed answers will not be given full marks. • show all working in your answers to numerical questions. No credit will be given for an incorrect answer unless it is accompanied by details of the working. • make sure chemical equations are balanced and that the formulas for individual substances ...
... unsimpliÞed answers will not be given full marks. • show all working in your answers to numerical questions. No credit will be given for an incorrect answer unless it is accompanied by details of the working. • make sure chemical equations are balanced and that the formulas for individual substances ...
Electromagnetic cascade in high energy electron, positron, and
... The processes typical for High Intensity Particle Physics [1], i.e., the interactions of charged particles with strong electromagnetic fields [2], have attracted considerable interest recently. This interest is due to the rapid growth of the maximum achievable laser intensity at many existing, const ...
... The processes typical for High Intensity Particle Physics [1], i.e., the interactions of charged particles with strong electromagnetic fields [2], have attracted considerable interest recently. This interest is due to the rapid growth of the maximum achievable laser intensity at many existing, const ...
Document
... moles to gain the molar ratios for each element in the compound. These are the formula subscripts. (X2Y3 etc…) Subscript for C ...
... moles to gain the molar ratios for each element in the compound. These are the formula subscripts. (X2Y3 etc…) Subscript for C ...
Structural Characterization of Surfactant
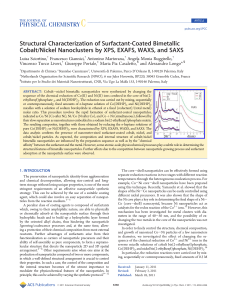
... This indicates that in our experimental conditions nickel is less prone to be oxidized than cobalt. The relevant presence of cobalt in the oxidized form of Co2þ in both the Ni/Co and Co/Ni samples confirms that this atomic species could quite easily migrate at the nanoparticle surface. For the follow ...
... This indicates that in our experimental conditions nickel is less prone to be oxidized than cobalt. The relevant presence of cobalt in the oxidized form of Co2þ in both the Ni/Co and Co/Ni samples confirms that this atomic species could quite easily migrate at the nanoparticle surface. For the follow ...
atomic theory and the periodic table
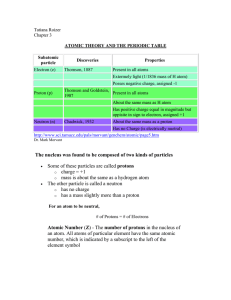
... can hold up to two electrons. (Encarta) When the a planet moves around the sun, you can plot a definite path for it which is called an orbit. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. The truth is different, and electrons in fact ...
... can hold up to two electrons. (Encarta) When the a planet moves around the sun, you can plot a definite path for it which is called an orbit. A simple view of the atom looks similar and you may have pictured the electrons as orbiting around the nucleus. The truth is different, and electrons in fact ...
Hydrogenic Rydberg atoms in strong magnetic fields: Theoretical
... one hand, high resolution experiments with highly excited one-eleclron systems (hydrogen and deuterium) have been performed in 4-6 T fields [15], and, on lhe other, numerical procedures bave been developed which allow an almost routine calculation of very accurate energy values. wave fu nctions, and ...
... one hand, high resolution experiments with highly excited one-eleclron systems (hydrogen and deuterium) have been performed in 4-6 T fields [15], and, on lhe other, numerical procedures bave been developed which allow an almost routine calculation of very accurate energy values. wave fu nctions, and ...
Electron-Positron Scattering
... At y = x, we would have to add an infinite (delta function) potential term to the Klein-Gordon equation, to represent the disturbance that creates the particle. This infinite potential term results in an infinite wavefunction at y = x, and indeed, DF (x − y) is infinite there. Vertices. The Feynman rule ...
... At y = x, we would have to add an infinite (delta function) potential term to the Klein-Gordon equation, to represent the disturbance that creates the particle. This infinite potential term results in an infinite wavefunction at y = x, and indeed, DF (x − y) is infinite there. Vertices. The Feynman rule ...
First Grade Science Pacing
... substance. Liquids are limited in the amount of dissolved solid or gas that they can contain. Aqueous solutions can be described by relative quantities of the dissolved substances and acidity or alkalinity (pH). The rate of a physical or chemical change may be affected by factors such as temperature ...
... substance. Liquids are limited in the amount of dissolved solid or gas that they can contain. Aqueous solutions can be described by relative quantities of the dissolved substances and acidity or alkalinity (pH). The rate of a physical or chemical change may be affected by factors such as temperature ...
Problems
... b. How the two boundary conditions reduce the number of acceptable solutions from two to one and limit the values of E that can be “allowed”. c. How the wave function is continuous even at the box boundaries, but dΨ/dx is not. In general dΨ/dx, which relates to the momentum because – i h d/dx is the ...
... b. How the two boundary conditions reduce the number of acceptable solutions from two to one and limit the values of E that can be “allowed”. c. How the wave function is continuous even at the box boundaries, but dΨ/dx is not. In general dΨ/dx, which relates to the momentum because – i h d/dx is the ...
Magneto-optical properties of charged excitons in quantum dots
... for 1 min.10 Ensemble photoluminescence 共PL兲 experiments show that the annealing step produces a bimodal distribution of dots: there are two dominant PL bands, one centered at 1.31 eV 共the red band兲 and one at 1.34 eV 共the blue band兲. A bimodal QD distribution has been observed before, notably in th ...
... for 1 min.10 Ensemble photoluminescence 共PL兲 experiments show that the annealing step produces a bimodal distribution of dots: there are two dominant PL bands, one centered at 1.31 eV 共the red band兲 and one at 1.34 eV 共the blue band兲. A bimodal QD distribution has been observed before, notably in th ...
Is Matter Made of Light? The Transluminal Energy Quantum (TEQ
... He also proposed that a particle of matter like the electron contains an amount of energy given by ...
... He also proposed that a particle of matter like the electron contains an amount of energy given by ...
The Classical Electrodynamics Approach to Explain
... cannot resolve the problem of the direction of motion of the liberated electron, and cannot explain the two and the muitl- photon photoelectric emission. From the above analysis, it is also realized that the photoelectric emission is dependent on the incident light's circular frequency ω and the pho ...
... cannot resolve the problem of the direction of motion of the liberated electron, and cannot explain the two and the muitl- photon photoelectric emission. From the above analysis, it is also realized that the photoelectric emission is dependent on the incident light's circular frequency ω and the pho ...
Redox Reactions - hrsbstaff.ednet.ns.ca
... Oxidation and reduction reaction = redox rxn Oxidation is loss of electrons and reduction is gain of electrons = transfer of electrons Those 2 reactions are occurring simultaneously ...
... Oxidation and reduction reaction = redox rxn Oxidation is loss of electrons and reduction is gain of electrons = transfer of electrons Those 2 reactions are occurring simultaneously ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.