Calculating molar volume
... The number of moles of vitamin C has to first be calculated. This is done indirectly by first working out the number of moles of iodine required and then using the redox equation to work out the number of moles of vitamin C present. Moles can then be converted to mass. Example: A 250 cm3 solution of ...
... The number of moles of vitamin C has to first be calculated. This is done indirectly by first working out the number of moles of iodine required and then using the redox equation to work out the number of moles of vitamin C present. Moles can then be converted to mass. Example: A 250 cm3 solution of ...
hong kong diploma of secondary education examination
... When ethanol from the breath of the driver is allowed to get into contact with electrode X, the following change occurs. CH3CH2OH CH3COOH Which of the following statements about the fuel cell are correct? (1) Electrode X acts as the anode of the cell. (2) Electrons flow from electrode X to electro ...
... When ethanol from the breath of the driver is allowed to get into contact with electrode X, the following change occurs. CH3CH2OH CH3COOH Which of the following statements about the fuel cell are correct? (1) Electrode X acts as the anode of the cell. (2) Electrons flow from electrode X to electro ...
Document
... 1. In thermodynamics it is the change in a certain function that is usually important. * Absolute values for H and G cannot be determined. 2. The third law of thermodynamics states that at 0 K, the entropy of a pure crystal is equal to 0. * Because this provides a starting point to compare all other ...
... 1. In thermodynamics it is the change in a certain function that is usually important. * Absolute values for H and G cannot be determined. 2. The third law of thermodynamics states that at 0 K, the entropy of a pure crystal is equal to 0. * Because this provides a starting point to compare all other ...
AP 3rd 9 weeks notes
... 1. In thermodynamics it is the change in a certain function that is usually important. * Absolute values for H and G cannot be determined. 2. The third law of thermodynamics states that at 0 K, the entropy of a pure crystal is equal to 0. * Because this provides a starting point to compare all other ...
... 1. In thermodynamics it is the change in a certain function that is usually important. * Absolute values for H and G cannot be determined. 2. The third law of thermodynamics states that at 0 K, the entropy of a pure crystal is equal to 0. * Because this provides a starting point to compare all other ...
CHEMICAL REACTIONS
... • Take one element at a time usually starting with the most complex substance. • It is usually better to balance in this order: metals, nonmetals, hydrogen, oxygen. • If everything balances except for O2, and there is no way to balance O2 with a whole number, use a fraction or mixed number. Then, mu ...
... • Take one element at a time usually starting with the most complex substance. • It is usually better to balance in this order: metals, nonmetals, hydrogen, oxygen. • If everything balances except for O2, and there is no way to balance O2 with a whole number, use a fraction or mixed number. Then, mu ...
doc - Dartmouth College
... the reaction. Unfortunately her balance was broken, but her pH meter was in working condition. She dissolved all her product in 1.00 L of water and found that the solution had a pH = 3.19. Calculate her yield, expressed as a percentage of her maximum yield from part (a). You will also need to know t ...
... the reaction. Unfortunately her balance was broken, but her pH meter was in working condition. She dissolved all her product in 1.00 L of water and found that the solution had a pH = 3.19. Calculate her yield, expressed as a percentage of her maximum yield from part (a). You will also need to know t ...
coordination compounds - Ahlcon Public School , Mayur Vihar Ph
... 33. When a mixture of NH 4Cl and K 2CrO7 are heated, stable colourless gas (A) was evolved which did not support combustion but magnesium continued to burn in it. The gas (A) reacted with calcium carbide in an electric furnanance forming a solid (B). The compound (B) was slowly hydrolysed by water f ...
... 33. When a mixture of NH 4Cl and K 2CrO7 are heated, stable colourless gas (A) was evolved which did not support combustion but magnesium continued to burn in it. The gas (A) reacted with calcium carbide in an electric furnanance forming a solid (B). The compound (B) was slowly hydrolysed by water f ...
Subject Area Assessment Guides
... DOK Level: [2] Analysis, Application The bonds in BaO are best described as(1) covalent, because valence electrons are shared (2) covalent, because valence electrons are transferred (3) ionic, because valence electrons are shared (4) ionic, because valence electrons are transferred ...
... DOK Level: [2] Analysis, Application The bonds in BaO are best described as(1) covalent, because valence electrons are shared (2) covalent, because valence electrons are transferred (3) ionic, because valence electrons are shared (4) ionic, because valence electrons are transferred ...
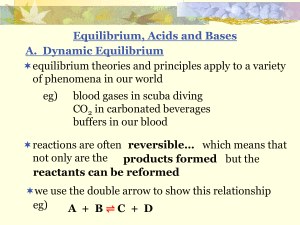
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.