Unit 14.1 REDOX Reactions Objectives REDOX Reactions
... • REDOX reactions can be used to convert chemical potential energy into electrical energy. ...
... • REDOX reactions can be used to convert chemical potential energy into electrical energy. ...
2.0 Chem 20 Final Review
... • The EN of the two atoms are quite different • The atom with the higher EN will remove one or more bonding e- from the other atom • Electron transfer occurs – Positive and negative ions are formed which electrically attract each other ...
... • The EN of the two atoms are quite different • The atom with the higher EN will remove one or more bonding e- from the other atom • Electron transfer occurs – Positive and negative ions are formed which electrically attract each other ...
BIG IDEAS - BC Curriculum - Province of British Columbia
... • Formulate physical or mental theoretical models to describe a phenomenon • Communicate scientific ideas, information, and perhaps a suggested course of action, for a specific purpose and audience, constructing evidence-based arguments and using appropriate scientific language, conventions, and rep ...
... • Formulate physical or mental theoretical models to describe a phenomenon • Communicate scientific ideas, information, and perhaps a suggested course of action, for a specific purpose and audience, constructing evidence-based arguments and using appropriate scientific language, conventions, and rep ...
1.Using the table above, decide if the element mercury (Hg) should
... b. Describe the bonding. What's bonded to what, and what kinds of bonds are involved (ionic, covalent, intermolecular forces, etc.)? The Si-O framework is an anion that can be a small or an extended structure. The Si-O framework forms an ionic compound with metal ions (except in the case of SiO2, wh ...
... b. Describe the bonding. What's bonded to what, and what kinds of bonds are involved (ionic, covalent, intermolecular forces, etc.)? The Si-O framework is an anion that can be a small or an extended structure. The Si-O framework forms an ionic compound with metal ions (except in the case of SiO2, wh ...
Chemical Equation Reactions
... 2. Solid calcium reacts with oxygen gas. 3. Solutions of aluminum chloride & sodium carbonate are mixed. 4. Liquid magnesium bromide is decomposed at high temperature. 5. Solid nickel is reacted with aqueous magnesium sulfate. 6. Chlorine gas is reacted with aqueous potassium bromide. 7. Solid magne ...
... 2. Solid calcium reacts with oxygen gas. 3. Solutions of aluminum chloride & sodium carbonate are mixed. 4. Liquid magnesium bromide is decomposed at high temperature. 5. Solid nickel is reacted with aqueous magnesium sulfate. 6. Chlorine gas is reacted with aqueous potassium bromide. 7. Solid magne ...
Triple Award - Cheltenham College
... Recall the charge on common ions – both metals and non-‐metals – and compound ions e.g. SO42-‐, CO32-‐ , NH4+ , NO3-‐ . Deduce the charge of an ion from the electronic configuration of the ...
... Recall the charge on common ions – both metals and non-‐metals – and compound ions e.g. SO42-‐, CO32-‐ , NH4+ , NO3-‐ . Deduce the charge of an ion from the electronic configuration of the ...
Quiz 1
... 7. Which of the following is a correct statement concerning solution A with a pH of 11.5 compared to solution B with a pH of 10.0? Solution A… a. has a smaller [OH¯] than solution B b. has a larger number of [H+] than solution B c. is more basic than solution B d. is more acidic than solution B e. h ...
... 7. Which of the following is a correct statement concerning solution A with a pH of 11.5 compared to solution B with a pH of 10.0? Solution A… a. has a smaller [OH¯] than solution B b. has a larger number of [H+] than solution B c. is more basic than solution B d. is more acidic than solution B e. h ...
Matter - GEOCITIES.ws
... filled with a gas under study. After connecting the electrodes to high voltage it was observed that when the pressure in the tube was decreased to 0.0001 atmospheres, the glass of the tube at the anode side begins to fluoroscence (emits light) when the rest of the tube is dark.. This shows that an i ...
... filled with a gas under study. After connecting the electrodes to high voltage it was observed that when the pressure in the tube was decreased to 0.0001 atmospheres, the glass of the tube at the anode side begins to fluoroscence (emits light) when the rest of the tube is dark.. This shows that an i ...
Review Worksheet
... e) There are no _________ forces between gas molecules or between molecules and the sides of the container with which they collide. In a real gas, there actually is attraction between the molecules of a gas. Once again, this attraction WILL BE IGNORED when discussing ideal gases. f) Molecules collid ...
... e) There are no _________ forces between gas molecules or between molecules and the sides of the container with which they collide. In a real gas, there actually is attraction between the molecules of a gas. Once again, this attraction WILL BE IGNORED when discussing ideal gases. f) Molecules collid ...
Chapter 3 : Simple Bonding Theory Why do they make chemical
... 3. More electronegative atoms should have negative FC. 4. FCs of opposite signs separated by large distance are unlikely. 5. The largest sum of the electronegativity differences for adjacent atoms. Ex: HOCl more stable than HClO ...
... 3. More electronegative atoms should have negative FC. 4. FCs of opposite signs separated by large distance are unlikely. 5. The largest sum of the electronegativity differences for adjacent atoms. Ex: HOCl more stable than HClO ...
E b
... Activity coefficient of an individual ion is a theoretical quantity. Ions exist in solutions only in combinations with oppositely charged co-ions. Therefore we cannot experimentally measure the activity coefficient of an individual ion. In experiment, we can only able to determinate the mean activi ...
... Activity coefficient of an individual ion is a theoretical quantity. Ions exist in solutions only in combinations with oppositely charged co-ions. Therefore we cannot experimentally measure the activity coefficient of an individual ion. In experiment, we can only able to determinate the mean activi ...
AP CHEMISTRY SUMMER ASSIGNMENT
... The United States is the last county in the industrial world that uses the English system. To be consistent with every other country, we are slowly adopting the International System or SI System. The SI System is based on the metric system. We see examples of the SI system when we go to the grocery ...
... The United States is the last county in the industrial world that uses the English system. To be consistent with every other country, we are slowly adopting the International System or SI System. The SI System is based on the metric system. We see examples of the SI system when we go to the grocery ...
Chemical Reactions
... Covalent Compounds • Covalent compounds are two nonmetals • Use prefixes to represent a number (tells how many atoms) • Study table on page 393 ...
... Covalent Compounds • Covalent compounds are two nonmetals • Use prefixes to represent a number (tells how many atoms) • Study table on page 393 ...
AP Chemistry Summer Packet ANSWERS
... b. A colorless, crystalline solid is decomposed, yielding a pale yellow-green gas and a soft, shiny metal. c. A cup of tea becomes sweeter as sugar is added to it. a. physical, mixture b. chemical, compound c. physical, mixture CHAPTER 2 1. Describe Dalton’s atomic theory. All matter is made up of a ...
... b. A colorless, crystalline solid is decomposed, yielding a pale yellow-green gas and a soft, shiny metal. c. A cup of tea becomes sweeter as sugar is added to it. a. physical, mixture b. chemical, compound c. physical, mixture CHAPTER 2 1. Describe Dalton’s atomic theory. All matter is made up of a ...
Here are the answers and work for your summer packet.
... b. A colorless, crystalline solid is decomposed, yielding a pale yellow-green gas and a soft, shiny metal. c. A cup of tea becomes sweeter as sugar is added to it. a. physical, mixture b. chemical, compound c. physical, mixture CHAPTER 2 1. Describe Dalton’s atomic theory. All matter is made up of a ...
... b. A colorless, crystalline solid is decomposed, yielding a pale yellow-green gas and a soft, shiny metal. c. A cup of tea becomes sweeter as sugar is added to it. a. physical, mixture b. chemical, compound c. physical, mixture CHAPTER 2 1. Describe Dalton’s atomic theory. All matter is made up of a ...
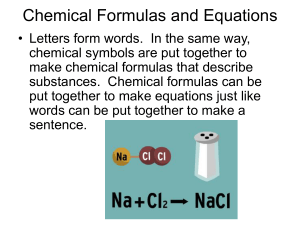
Chemical Formulas and Equations
... chemical symbols are put together to make chemical formulas that describe substances. Chemical formulas can be put together to make equations just like words can be put together to make a sentence. ...
... chemical symbols are put together to make chemical formulas that describe substances. Chemical formulas can be put together to make equations just like words can be put together to make a sentence. ...
Presentation
... They are the smallest particle of a substance that still retains the properties of that substance and is composed of 2 or more atoms. ...
... They are the smallest particle of a substance that still retains the properties of that substance and is composed of 2 or more atoms. ...
AQA GCSE Chemistry My Revision Notes
... (c) There are some advantages of drinking hard water. Give one of them. (1 mark) (d) What happens if you use temporarily hard water in a kettle? (2 marks) (e) Explain how an ion-exchange column softens hard water. (2 marks) (f) Another way of softening hard water is to use sodium carbonate. Explain ...
... (c) There are some advantages of drinking hard water. Give one of them. (1 mark) (d) What happens if you use temporarily hard water in a kettle? (2 marks) (e) Explain how an ion-exchange column softens hard water. (2 marks) (f) Another way of softening hard water is to use sodium carbonate. Explain ...
SC71 Chemistry
... Concept 2: Scientific Testing (Investigating and Modeling) Design and conduct controlled investigations. PO 1. Demonstrate safe and ethical procedures (e.g., use and care of technology, materials, organisms) and behavior in all science inquiry. PO 2. Identify the resources needed to conduct an inves ...
... Concept 2: Scientific Testing (Investigating and Modeling) Design and conduct controlled investigations. PO 1. Demonstrate safe and ethical procedures (e.g., use and care of technology, materials, organisms) and behavior in all science inquiry. PO 2. Identify the resources needed to conduct an inves ...
Single crystal structure determination using synchrotron X
... TFE/dimethyl sulfoxide (DMSO) (60/40 v/v%) mixed solvent (700 μ L) and heated at 100°C (Fig. 2). The time-dependent NMR monitoring of the reaction solution revealed that the self-assembly completed after 24 h. The formation of a giant product was first indicated by the broadness of the 1H NMR signal ...
... TFE/dimethyl sulfoxide (DMSO) (60/40 v/v%) mixed solvent (700 μ L) and heated at 100°C (Fig. 2). The time-dependent NMR monitoring of the reaction solution revealed that the self-assembly completed after 24 h. The formation of a giant product was first indicated by the broadness of the 1H NMR signal ...
Document
... Are attractive forces in which hydrogen that is covalently bonded to a very electronegative atom is also weakly bonded to an unshared electron pair of another ...
... Are attractive forces in which hydrogen that is covalently bonded to a very electronegative atom is also weakly bonded to an unshared electron pair of another ...
3(aq)
... slippery to the touch (like soap) 1. are also called “alkaline” solutions 2. they are substances that produce hydroxide ions (OH-) when dissolved into water. 3. bases are considered “strong” bases are those that easily dissolve when placed into water, forming electrolytes (charged ion particles) in ...
... slippery to the touch (like soap) 1. are also called “alkaline” solutions 2. they are substances that produce hydroxide ions (OH-) when dissolved into water. 3. bases are considered “strong” bases are those that easily dissolve when placed into water, forming electrolytes (charged ion particles) in ...
Electronic Structure and the Periodic Table
... than one electron is involved. Effective nuclear charge(kernel charge) Inner electrons act to shield outer ones from the positive charge of the nucleus. Some orbitals penetrate to the nucleus more than others: s > p > d > f ...
... than one electron is involved. Effective nuclear charge(kernel charge) Inner electrons act to shield outer ones from the positive charge of the nucleus. Some orbitals penetrate to the nucleus more than others: s > p > d > f ...
Chemistry Midterm Review
... Matter can be classified as either: - ________ _________ which cannot be separated into simpler substances by physical methods. Or - _____________ which can be separated into simpler substances by physical methods such as filtering, settling, evaporating, boiling, etc.. Pure Substances can be classi ...
... Matter can be classified as either: - ________ _________ which cannot be separated into simpler substances by physical methods. Or - _____________ which can be separated into simpler substances by physical methods such as filtering, settling, evaporating, boiling, etc.. Pure Substances can be classi ...
Interactive comment on “On the composition of ammonia
... Title: “On the composition of ammonia-sulfuric acid clusters during aerosol particle formation” This title is a bit misleading. The clusters that were observed in this study are ions and not electrically neutral clusters. Consider adding the word “ions” in the title to remove confusion. Line 15 pg 1 ...
... Title: “On the composition of ammonia-sulfuric acid clusters during aerosol particle formation” This title is a bit misleading. The clusters that were observed in this study are ions and not electrically neutral clusters. Consider adding the word “ions” in the title to remove confusion. Line 15 pg 1 ...