Electromagnetic Spectrum activity
... An atom with a half filled or completely filled sublevels are also stable. Example: Cr (Z=24) has a configuration of [Ar] 4s13d5. Here the d orbital is half filled. And, Cu (Z=29) has a configuration of [Ar]4s13d10. Here the d orbital is completely filled. Note, we would have expected a configuratio ...
... An atom with a half filled or completely filled sublevels are also stable. Example: Cr (Z=24) has a configuration of [Ar] 4s13d5. Here the d orbital is half filled. And, Cu (Z=29) has a configuration of [Ar]4s13d10. Here the d orbital is completely filled. Note, we would have expected a configuratio ...
ATOMIC STRUCTURE NOTES n hcZ E ℜ
... through the nucleus. Therefore, it is more shielded from the nucleus by the electrons of the core. We can conclude that a 2s electron has lower energy (more bound) than a 2p, so for Lithium the ground state configuration must be 1s22s1. It usually follows that: ns > np > nd > nf ...
... through the nucleus. Therefore, it is more shielded from the nucleus by the electrons of the core. We can conclude that a 2s electron has lower energy (more bound) than a 2p, so for Lithium the ground state configuration must be 1s22s1. It usually follows that: ns > np > nd > nf ...
Lecture notes, part 1
... Notice that both the wave equation and its solutions ψ(x, t) are complex. The wave equation is called the Schroedinger Wave Equation (1926). It is valid for particles in free space only (no forces acting). In 3d, it has the form i~ ...
... Notice that both the wave equation and its solutions ψ(x, t) are complex. The wave equation is called the Schroedinger Wave Equation (1926). It is valid for particles in free space only (no forces acting). In 3d, it has the form i~ ...
Chapter 6 Outline full
... A collection of orbitals with the same value of n is called an electron shell. • There are n2 orbitals in a shell described by a the n value. • For example, for n = 3, there are 32 = 9 orbitals. • A set of orbitals with the same n and l is called a subshell. • Each subshell is designated by a number ...
... A collection of orbitals with the same value of n is called an electron shell. • There are n2 orbitals in a shell described by a the n value. • For example, for n = 3, there are 32 = 9 orbitals. • A set of orbitals with the same n and l is called a subshell. • Each subshell is designated by a number ...
Document
... A line spectrum shows only certain colors or specific wavelengths of light. When atoms are heated, they emit light. This process produces a line spectrum that is specific to that atom. The emission spectra of six elements are shown on the next slide. ...
... A line spectrum shows only certain colors or specific wavelengths of light. When atoms are heated, they emit light. This process produces a line spectrum that is specific to that atom. The emission spectra of six elements are shown on the next slide. ...
the constancy of for an ideal gas undergoing an adiabatic
... In an ideal gas, all molecules are considered independently from each other. As we will see, this can be checked out. Thus, Eq.(7) remains valid, even if the gas consists in just one molecule. And once again, within the frame of the Kinetic Theory of Gases, one first, is to express the pressure for ...
... In an ideal gas, all molecules are considered independently from each other. As we will see, this can be checked out. Thus, Eq.(7) remains valid, even if the gas consists in just one molecule. And once again, within the frame of the Kinetic Theory of Gases, one first, is to express the pressure for ...
The Quantum mechanical model of the atom
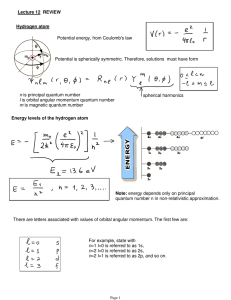
... atoms. n = principle quantum number = orbital’s energy level and relative size l = describe orbital’s shape (subshell) ml = describe orbital’s orientation in space (magnetic quantum number). ms= describes behaviour of a specific electron in an orbital (spin quantum number). ...
... atoms. n = principle quantum number = orbital’s energy level and relative size l = describe orbital’s shape (subshell) ml = describe orbital’s orientation in space (magnetic quantum number). ms= describes behaviour of a specific electron in an orbital (spin quantum number). ...
Special Issue on Lie Group Representation Theory, Coherent States,
... has not progressed very far, except for some important achievements in one and two dimensions (mainly: Virasoro, W ∞ and Kac-Moody symmetries, in connection with conformal invariant quantum field theories), and necessary breakthroughs in the subject remain to be carried out. Researchers in the field ...
... has not progressed very far, except for some important achievements in one and two dimensions (mainly: Virasoro, W ∞ and Kac-Moody symmetries, in connection with conformal invariant quantum field theories), and necessary breakthroughs in the subject remain to be carried out. Researchers in the field ...
Quantum Physics in a Nutshell
... • Planck studied the emission spectrums of incandescent objects in greater detail and made the following conclusions: • The energy of electromagnetic radiation was directly related to frequency. Higher frequency waves have more energy. • Any spectral line (frequency) can vary in intensity (energy). ...
... • Planck studied the emission spectrums of incandescent objects in greater detail and made the following conclusions: • The energy of electromagnetic radiation was directly related to frequency. Higher frequency waves have more energy. • Any spectral line (frequency) can vary in intensity (energy). ...
Semiconductor Nanocrystals
... Why they work - Bohr exciton radius • Roughly follows particle in a box model • In bulk CdSe, absorption of a photon creates an electron hole pair • Electron and hole maintain a characteristic distance known as the bulk Bohr exciton radius. This value depends on the material properties. • For CdSe, ...
... Why they work - Bohr exciton radius • Roughly follows particle in a box model • In bulk CdSe, absorption of a photon creates an electron hole pair • Electron and hole maintain a characteristic distance known as the bulk Bohr exciton radius. This value depends on the material properties. • For CdSe, ...
Particle in a box

In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example a ball trapped inside a large box, the particle can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never ""sit still"". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes.The particle in a box model provides one of the very few problems in quantum mechanics which can be solved analytically, without approximations. This means that the observable properties of the particle (such as its energy and position) are related to the mass of the particle and the width of the well by simple mathematical expressions. Due to its simplicity, the model allows insight into quantum effects without the need for complicated mathematics. It is one of the first quantum mechanics problems taught in undergraduate physics courses, and it is commonly used as an approximation for more complicated quantum systems.